Answered step by step
Verified Expert Solution
Question
1 Approved Answer
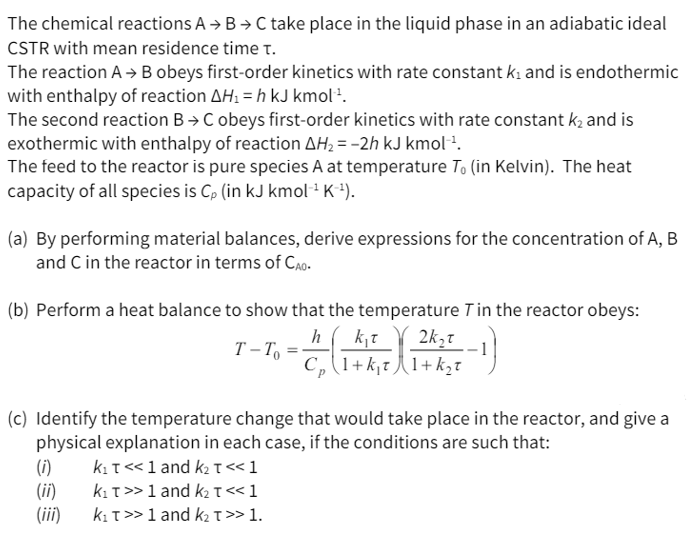
The chemical reactions A B C take place in the liquid phase in an adiabatic ideal CSTR with mean residence time . The reaction A
The chemical reactions take place in the liquid phase in an adiabatic ideal
CSTR with mean residence time
The reaction obeys firstorder kinetics with rate constant and is endothermic
with enthalpy of reaction
The second reaction obeys firstorder kinetics with rate constant and is
exothermic with enthalpy of reaction
The feed to the reactor is pure species at temperature in Kelvin The heat
capacity of all species is in
a By performing material balances, derive expressions for the concentration of
and in the reactor in terms of
b Perform a heat balance to show that the temperature in the reactor obeys:
c Identify the temperature change that would take place in the reactor, and give a
physical explanation in each case, if the conditions are such that:
i and
ii and
iii and
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


