Question
The concentration of various cations in seawater in moles per liter are: Na+ = 0.46 M Mg+ = 0.056 M Ca+ = 0.01 M
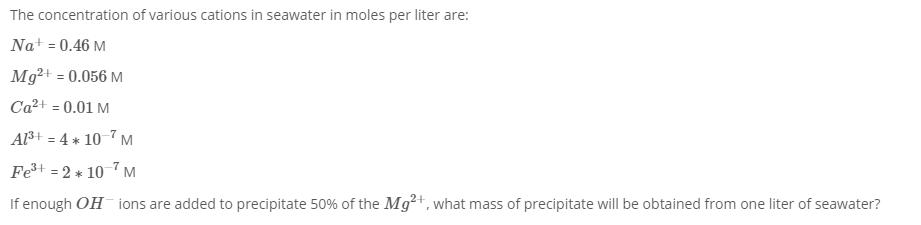
The concentration of various cations in seawater in moles per liter are: Na+ = 0.46 M Mg+ = 0.056 M Ca+ = 0.01 M Al+ = 4 * 10 7 M Fe+ = 2 * 10 M If enough OH ions are added to precipitate 50% of the Mg+, what mass of precipitate will be obtained from one liter of seawater?
Step by Step Solution
3.43 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1
Recall the expression for solubility product co...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introduction To Probability And Statistics
Authors: William Mendenhall, Robert Beaver, Barbara Beaver
14th Edition
1133103758, 978-1133103752
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



