Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The copper in an aqueous sample was determined by AAS. First, 19.0 mL of unknown were pipetted into each of five 50.0 mL volumetric flask.
The copper in an aqueous sample was determined by AAS. First, 19.0 mL of unknown were pipetted into each of five 50.0 mL volumetric flask. Various volumes of standard containing 12.2 ppm Cu were added to the flasks and the solution were then diluted to volume. 1. Calculate the concentration of standard Cu in each flask 2. Plot the graph of absorbance vs concentration of standard 3. Determine the linear equation of the data 4. Find the concentration of the sample based on the equation obtain
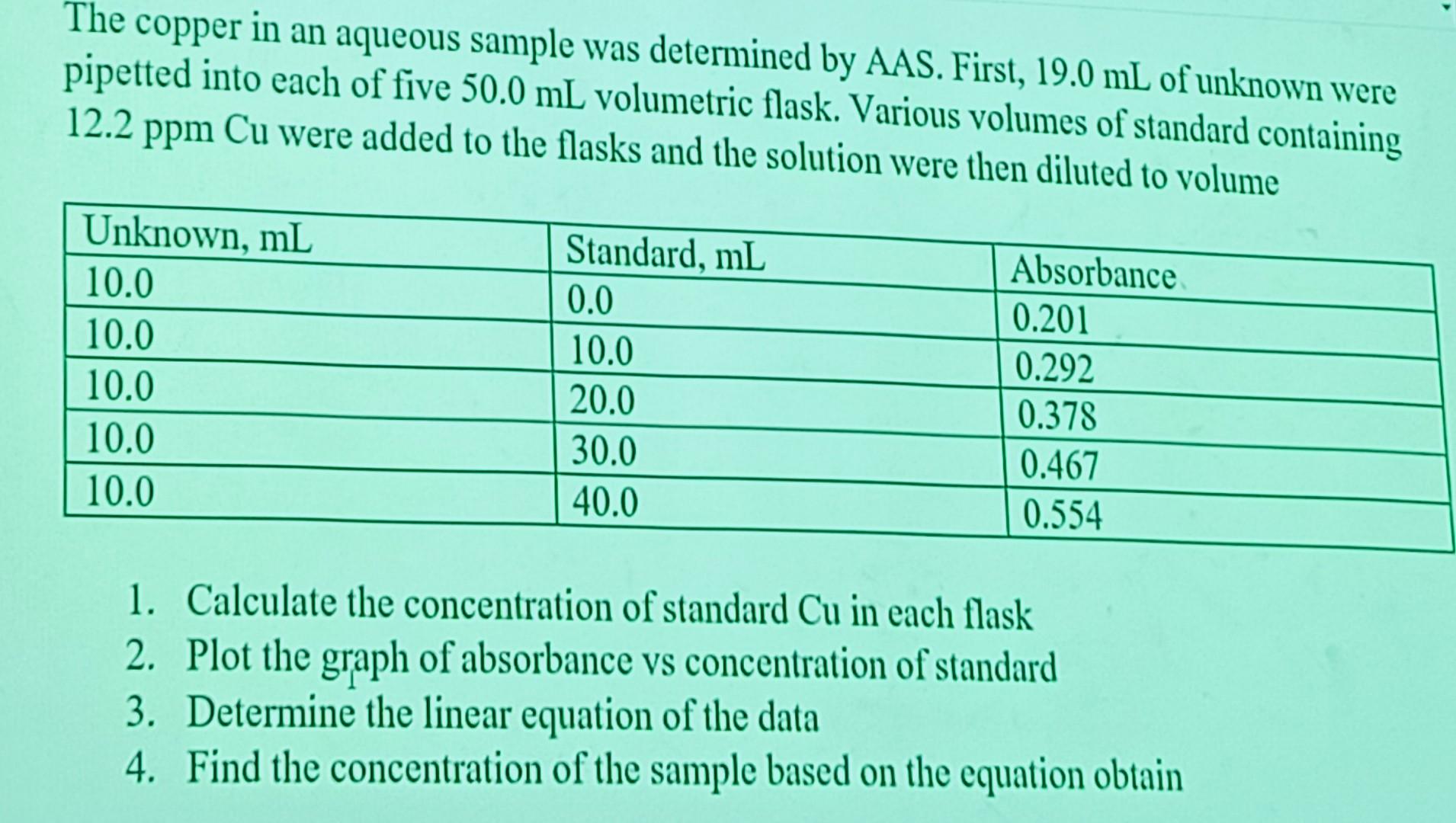
The copper in an aqueous sample was determined by AAS. First, 19.0mL of unknown were pipetted into each of five 50.0mL volumetric flask. Various volumes of standard containing 12.2ppmCu were added to the flasks and the solution were then diluted to volume 1. Calculate the concentration of standard Cu in each flask 2. Plot the graph of absorbance vs concentration of standard 3. Determine the linear equation of the data 4. Find the concentration of the sample based on the equation obtain
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


