Question
The data below were collected for the following reaction: CH3CI(g)+3C12(g)?CC14(g)+3HCl(g) A) Calculate the value of the rate constant, k B)What is the overall order
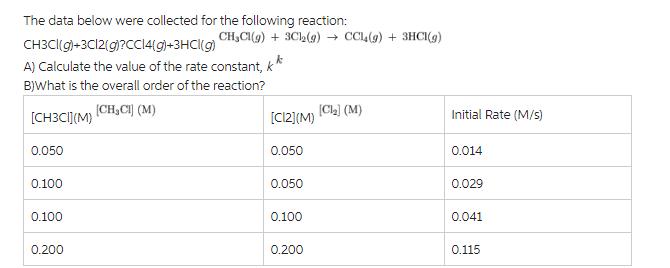
The data below were collected for the following reaction: CH3CI(g)+3C12(g)?CC14(g)+3HCl(g) A) Calculate the value of the rate constant, k B)What is the overall order of the reaction? (CH,Cl] (M) [CH3CI](M) 0.050 0.100 0.100 0.200 CHCl(g) + 3Cl(g) CCL (g) + 3HCI(g) [C12](M) 0.050 0.050 0.100 0.200 [Ch2] (M) Initial Rate (M/s) 0.014 0.029 0.041 0.115
Step by Step Solution
3.47 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Statistics Principles And Methods
Authors: Richard A. Johnson, Gouri K. Bhattacharyya
7th Edition
8126557745, 470904119, 978-0470904114
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



