Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The following data was collected after running a calorimetric experiment using three salts. Table 1: AHsol Values of Salts Salt AHsol (kJ/mol) Lil LINO3
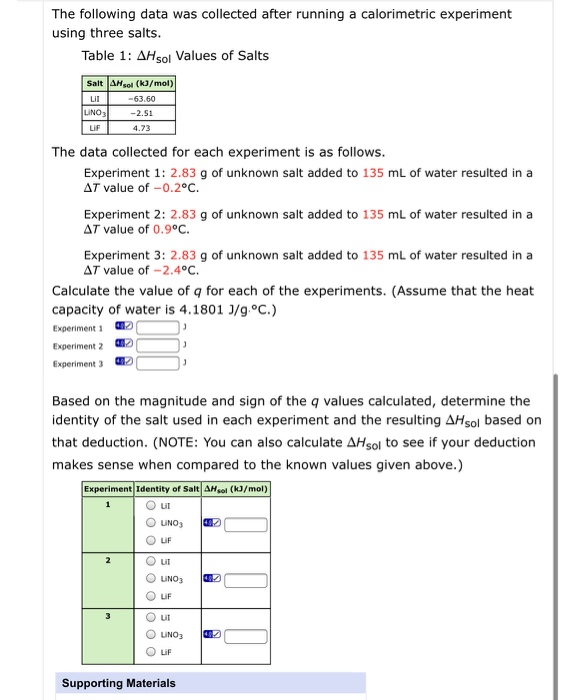
The following data was collected after running a calorimetric experiment using three salts. Table 1: AHsol Values of Salts Salt AHsol (kJ/mol) Lil LINO3 -63.60 -2.51 LIF 4.73 The data collected for each experiment is as follows. Experiment 1: 2.83 g of unknown salt added to 135 ml of water resulted in a AT value of -0.2C. Experiment 2: 2.83 g of unknown salt added to 135 mL of water resulted in a AT value of 0.9c. Experiment 3: 2.83 g of unknown salt added to 135 mL of water resulted in a AT value of -2.4c. Calculate the value of q for each of the experiments. (Assume that the heat capacity of water is 4.1801 J/g.C.) Experiment 1 Experiment 2 Experiment 3 Based on the magnitude and sign of the q values calculated, determine the identity of the salt used in each experiment and the resulting AHsol based on that deduction. (NOTE: You can also calculate AHsol to see if your deduction makes sense when compared to the known values given above.) Experiment Identity of Salt AHsol (k/mol) LINO3 LIF O ut O UNO3 LIF O ut O UNO3 Lif Supporting Materials O O 0 O O
Step by Step Solution
★★★★★
3.52 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1
First of all Convert Ansol of each salt om KJmof to ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


