Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The decomposition of cyclopropane to propene is a unimolecular reaction. It can be studied over a range of pressures. The following data gives values of
The decomposition of cyclopropane to propene is a unimolecular reaction. It can be studied over a range of pressures. The following data gives values of values of kit over a range of concentrations. Note: PV = nRT, hence pressure is proportional to concentration.
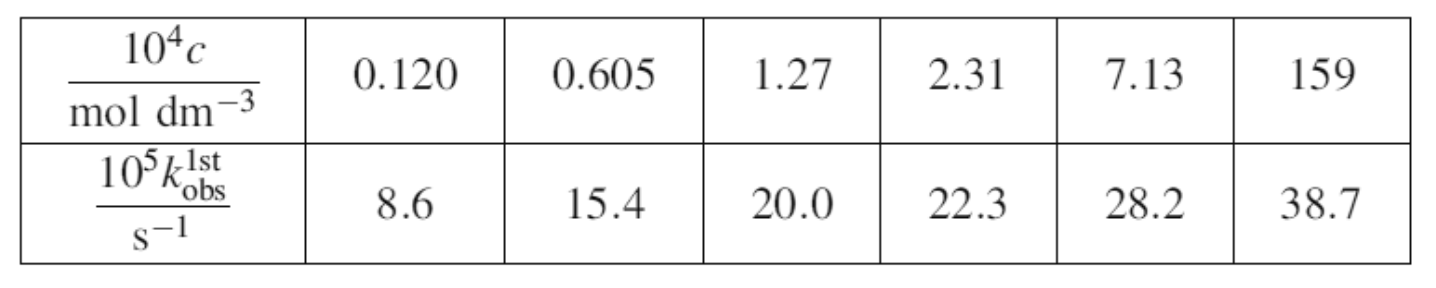
(a) Plot versus concentration and comment on the shape of the graph.
(b) From the data above, find [A]1/2 and show this to equal k2/k-1.
(c) Find k2 and then k1 , taking Z=5x1010 mol-1 dm3 s-1 , and =1.
(d) Why can k-1 be taken to equal a typical value of Z, the collision number?
104c mol dm -3 105 kl 1st obs 8-1 0.120 8.6 0.605 15.4 1.27 2.31 20.0 22.3 7.13 28.2 159 38.7Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


