Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The decomposition of N2O4 is given by the reaction N2O42NO2 For this reaction, G=64,709RT[1.696lnT+0.0665103T+T20.602105+33.08] the reaction takes place at 350K and the feed consists of
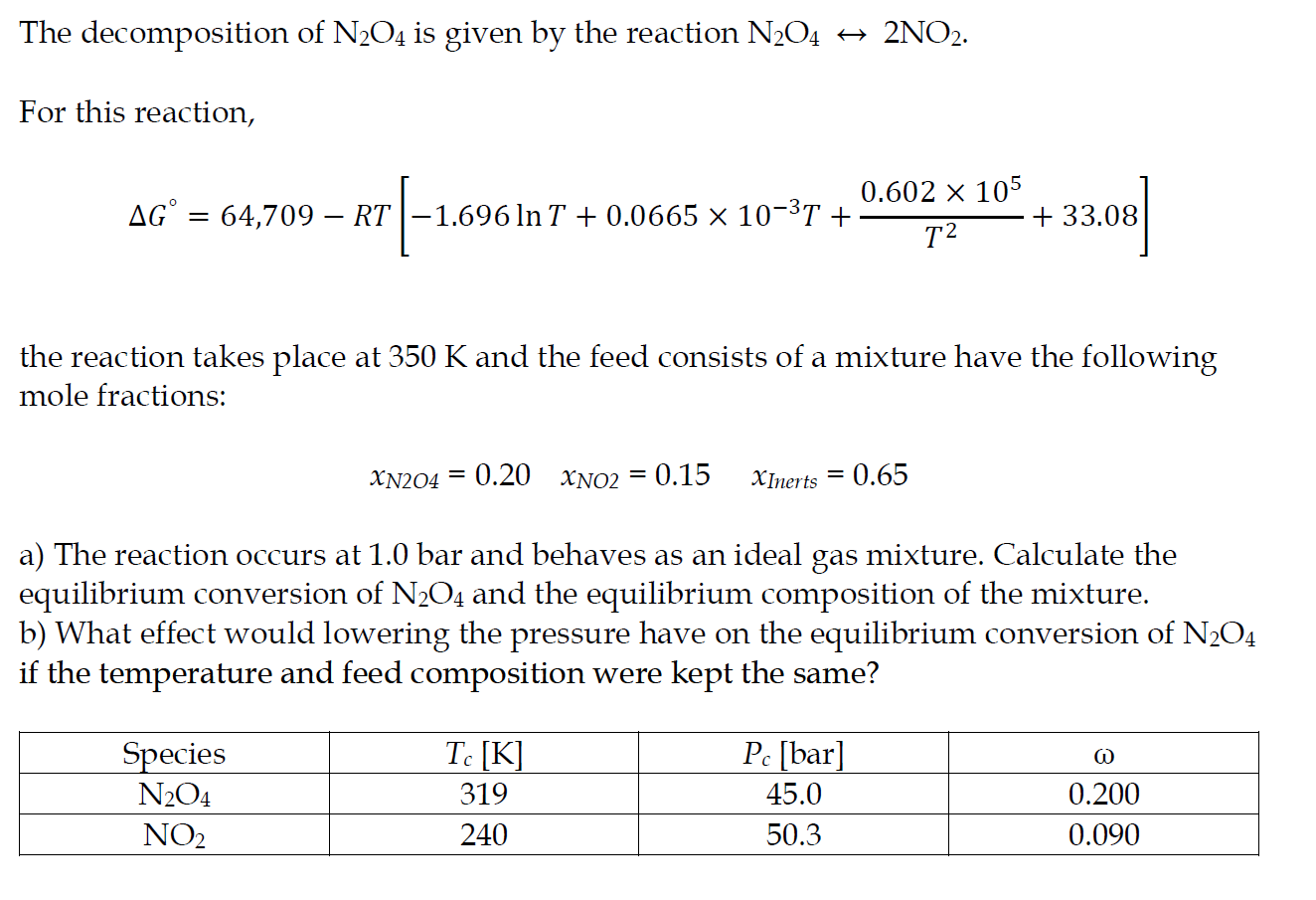 The decomposition of N2O4 is given by the reaction N2O42NO2 For this reaction, G=64,709RT[1.696lnT+0.0665103T+T20.602105+33.08] the reaction takes place at 350K and the feed consists of a mixture have the following mole fractions: xN2O4=0.20xNO2=0.15xInerts=0.65 a) The reaction occurs at 1.0 bar and behaves as an ideal gas mixture. Calculate the equilibrium conversion of N2O4 and the equilibrium composition of the mixture. b) What effect would lowering the pressure have on the equilibrium conversion of N2O4 if the temperature and feed composition were kept the same
The decomposition of N2O4 is given by the reaction N2O42NO2 For this reaction, G=64,709RT[1.696lnT+0.0665103T+T20.602105+33.08] the reaction takes place at 350K and the feed consists of a mixture have the following mole fractions: xN2O4=0.20xNO2=0.15xInerts=0.65 a) The reaction occurs at 1.0 bar and behaves as an ideal gas mixture. Calculate the equilibrium conversion of N2O4 and the equilibrium composition of the mixture. b) What effect would lowering the pressure have on the equilibrium conversion of N2O4 if the temperature and feed composition were kept the same Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


