Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The decomposition of SO,C1, is first order in SO2Cl2 and has a rate constant of 1.40x10-4 5-1 at a certain temperature. S h Part A

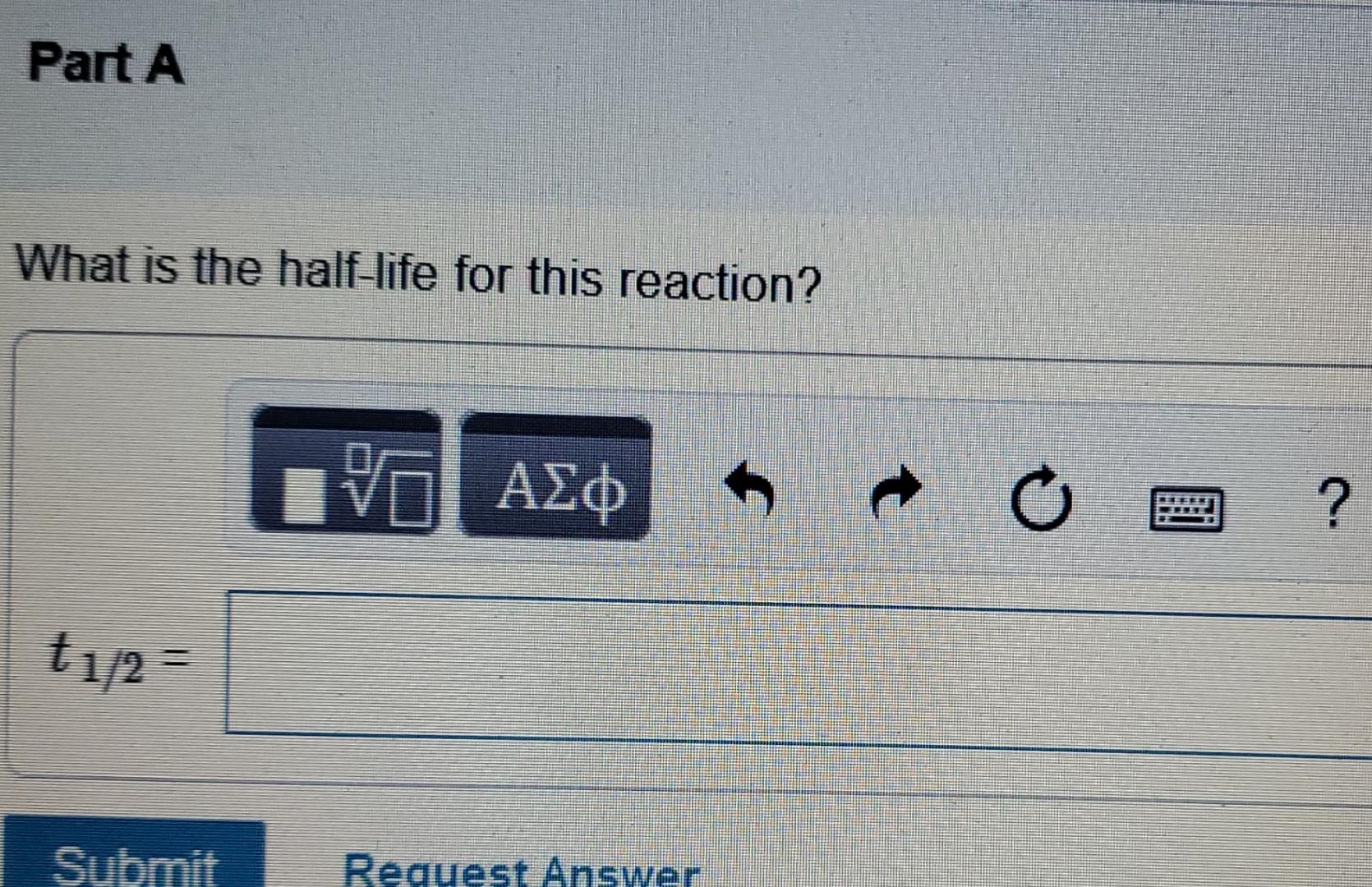
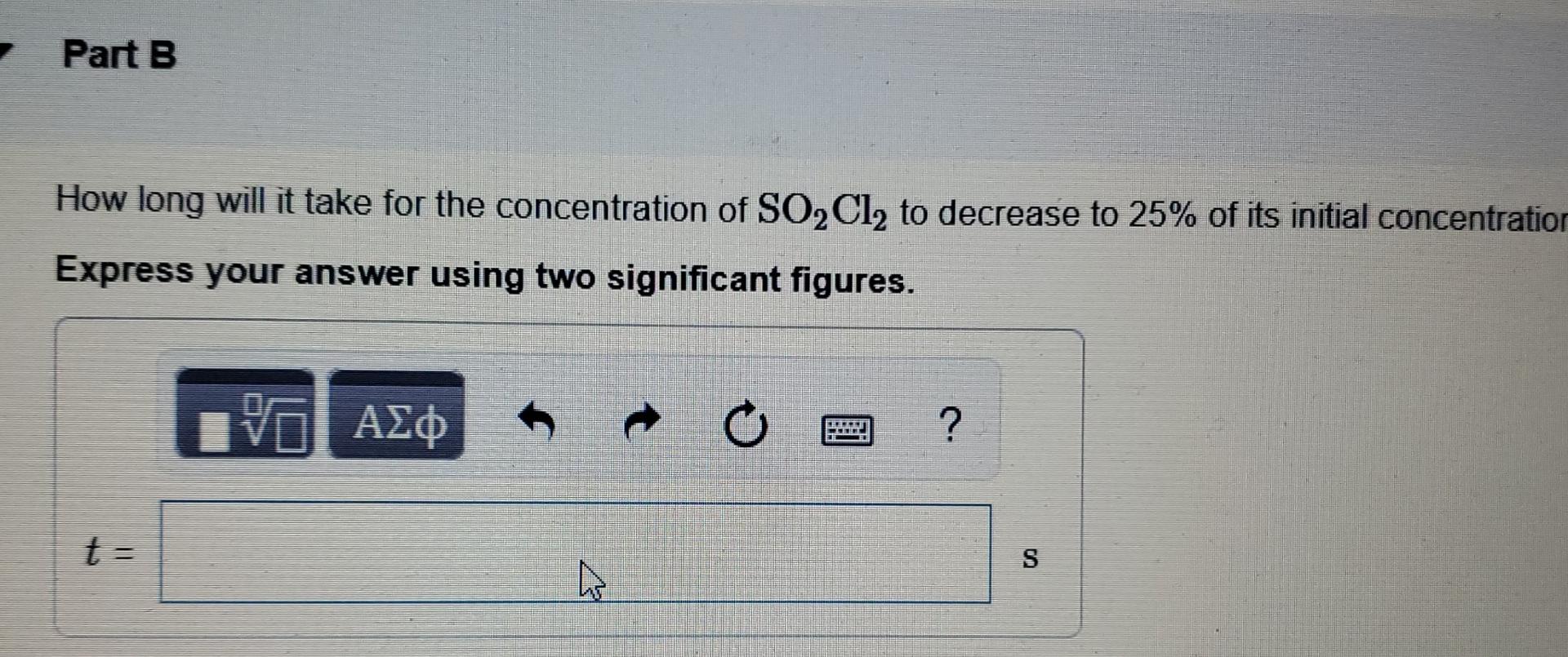
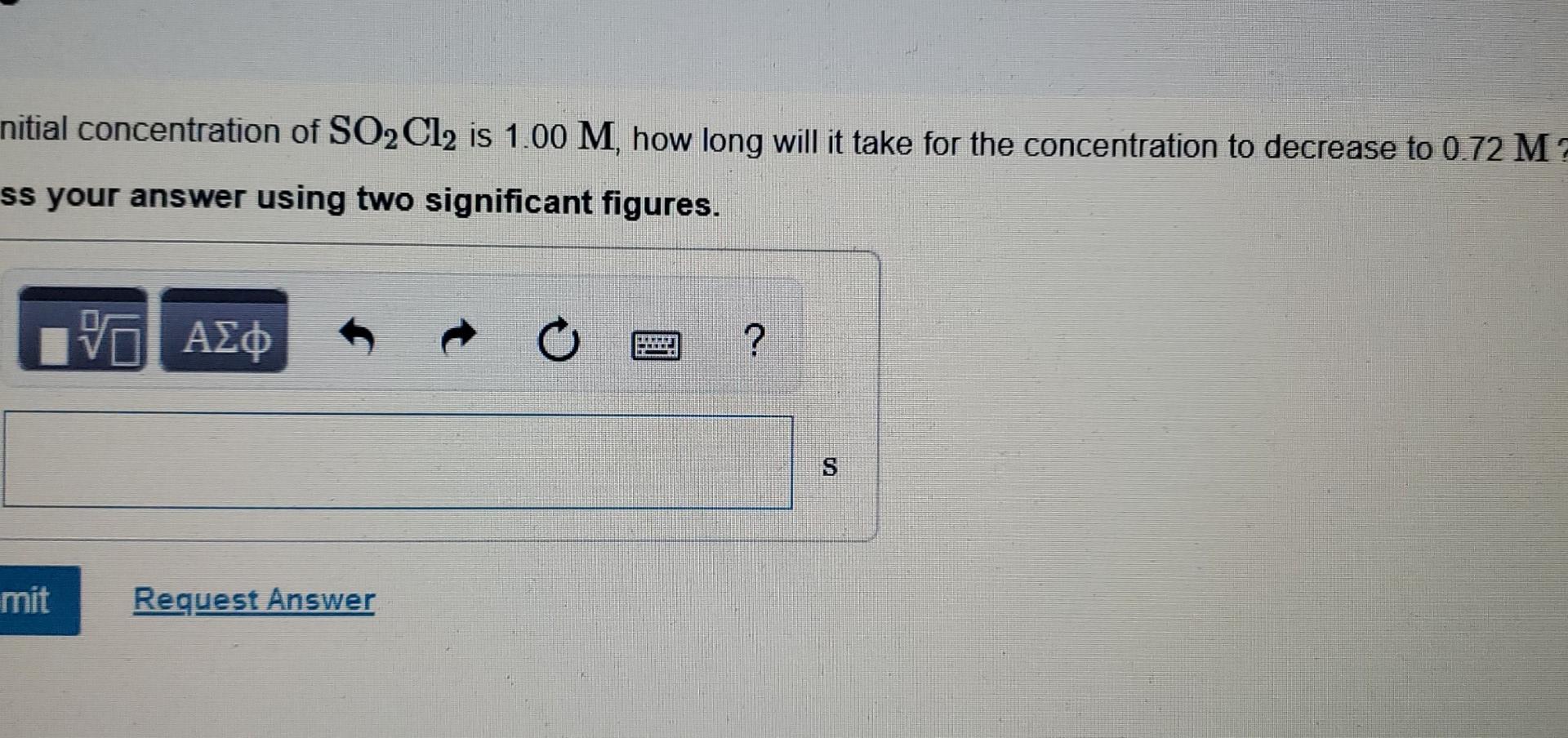
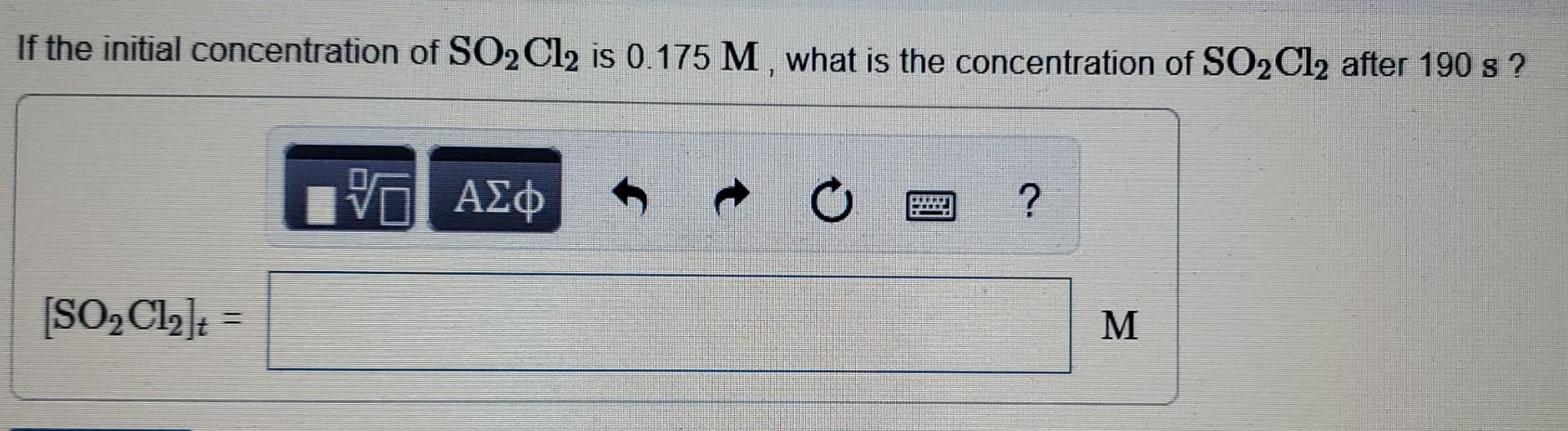
The decomposition of SO,C1, is first order in SO2Cl2 and has a rate constant of 1.40x10-4 5-1 at a certain temperature. S h Part A What is the half-life for this reaction? t O ? t1/2 = Submit Request Answer Part B How long will it take for the concentration of SO2Cl2 to decrease to 25% of its initial concentration Express your answer using two significant figures. 0 ? 0 nitial concentration of SO2Cl2 is 1.00 M, how long will it take for the concentration to decrease to 0.72 M? ss your answer using two significant figures. TE ? s mit Request Answer If the initial concentration of SO2Cl2 is 0.175 M , what is the concentration of SO2Cl2 after 190 s? vo ? [SO2Cl2] = M
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


