The dehydration butanol of alumina is carried out over a silica-alumina catalyst at 680K. CH3CH2CH2CH20H------->cat CH3CH=CHCH3 + H2O The rate law is -r Bu
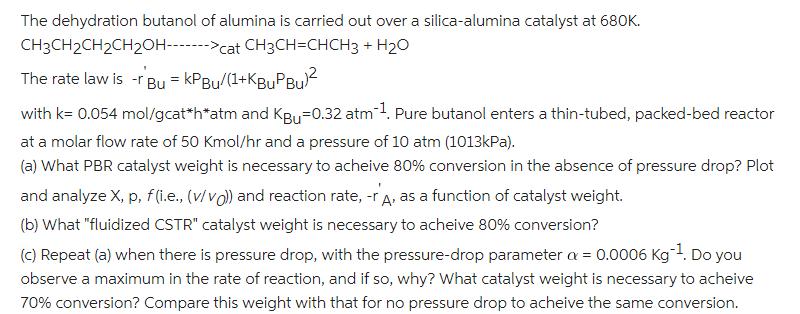
The dehydration butanol of alumina is carried out over a silica-alumina catalyst at 680K. CH3CH2CH2CH20H------->cat CH3CH=CHCH3 + H2O The rate law is -r Bu = KPBU/(1+KBuPBul with k= 0.054 mol/gcat*h*atm and KBu=0.32 atm1. Pure butanol enters a thin-tubed, packed-bed reactor at a molar flow rate of 50 Kmol/hr and a pressure of 10 atm (1013kPa). (a) What PBR catalyst weight is necessary to acheive 80% conversion in the absence of pressure drop? Plot and analyze X, p, f (i.e., (v/vo) and reaction rate, -r A, as a function of catalyst weight. (b) What "fluidized CSTR" catalyst weight is necessary to acheive 80% conversion? (C) Repeat (a) when there is pressure drop, with the pressure-drop parameter a = 0.0006 Kg1. Do you observe a maximum in the rate of reaction, and if so, why? What catalyst weight is necessary to acheive 70% conversion? Compare this weight with that for no pressure drop to acheive the same conversion.
Step by Step Solution
3.43 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
635f36f4962d7_231192.pdf
180 KBs PDF File
635f36f4962d7_231192.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


