Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For problems 2 & 3, the density of the sample is 0.9977 g/mL and the molar mass of calcium carbonate is 100.0 g/mol. 2.
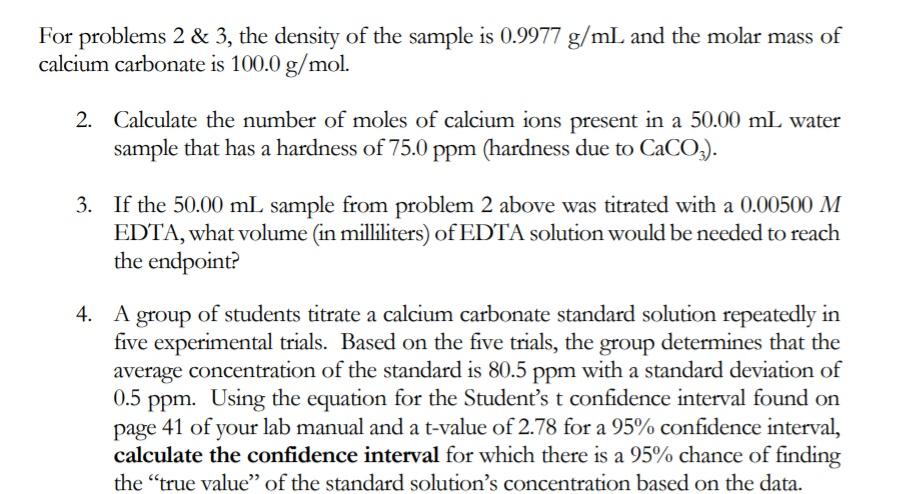
For problems 2 & 3, the density of the sample is 0.9977 g/mL and the molar mass of calcium carbonate is 100.0 g/mol. 2. Calculate the number of moles of calcium ions present in a 50.00 mL water sample that has a hardness of 75.0 ppm (hardness due to CaCO,). 3. If the 50.00 mL sample from problem 2 above was titrated with a 0.00500 M EDTA, what volume (in milliliters) of EDTA solution would be needed to reach the endpoint? 4. A of students titrate a calcium carbonate standard solution repeatedly in group five experimental trials. Based on the five trials, the group determines that the average concentration of the standard is 80.5 ppm with a standard deviation of 0.5 ppm. Using the equation for the Student's t confidence interval found on page 41 of your lab manual and a t-value of 2.78 for a 95% confidence interval, calculate the confidence interval for which there is a 95% chance of finding the "true value" of the standard solution's concentration based on the data.
Step by Step Solution
★★★★★
3.40 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
ANSWERS 2 There are 075 moles of calcium ions present in the sample 3 It would take 375 mL of EDTA s...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


