Question
The diffusivity of chloroform (CHC13) in the air at 298 K and 100 Kpa is measured by Winkelmann's method. The fall in level is
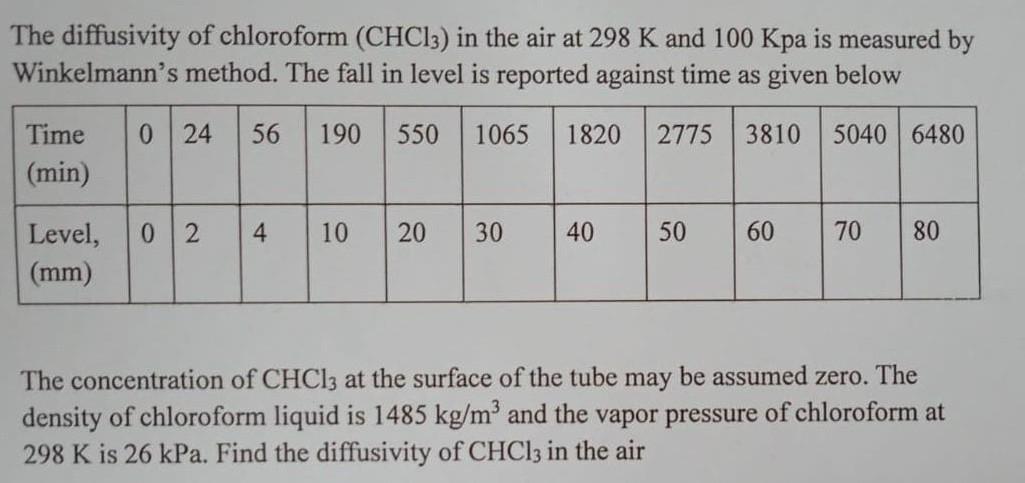
The diffusivity of chloroform (CHC13) in the air at 298 K and 100 Kpa is measured by Winkelmann's method. The fall in level is reported against time as given below 0 24 56 190 550 1065 1820 2775 3810 Time (min) Level, (mm) 02 4 10 20 30 40 50 60 5040 6480 70 80 The concentration of CHCl3 at the surface of the tube may be assumed zero. The density of chloroform liquid is 1485 kg/m and the vapor pressure of chloroform at 298 K is 26 kPa. Find the diffusivity of CHCl3 in the air
Step by Step Solution
3.45 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
To solve this problem we need to use the Winkelmanns method which is a technique used to measure the ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Income Tax Fundamentals 2013
Authors: Gerald E. Whittenburg, Martha Altus Buller, Steven L Gill
31st Edition
1111972516, 978-1285586618, 1285586611, 978-1285613109, 978-1111972516
Students also viewed these Law questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



