Answered step by step
Verified Expert Solution
Question
1 Approved Answer
the discussion section, just before the procedure, for a discussion of this calculation. We will be doing dilution calculations in almost every experiment in this
the discussion section, just before the procedure, for a discussion of this calculation. We will be doing dilution calculations in almost every experiment in this course.
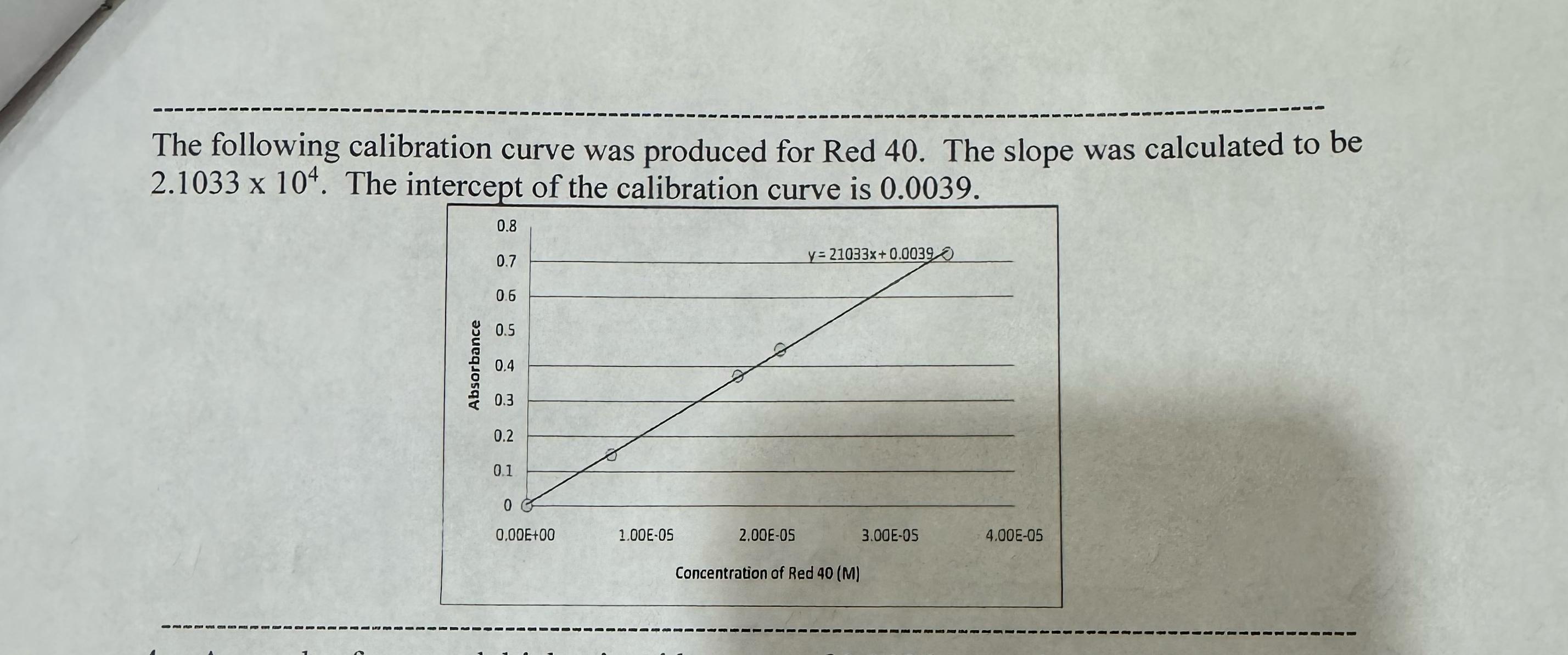
The solution described in problem # is placed in the spectrophotometer. Based on the calibration curve shown on the next page, what would you predict the absorbance to be Note: like the example given in the discussion, the absorbance should be calculated, not just estimated from the graph. Notice that the slope and intercept of the calibration curve are provided in the figure on the next page.
Aborbance
Absorbance
The following calibration curve was produced for Red The slope was calculated to be The intercept of the calibration curve is
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


