Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The electronic absorption spectrum of copper ammine complexes (i) Draw the electron distribution in the d orbitals of an octahedral Cu(II) complex and show the
The electronic absorption spectrum of copper ammine complexes
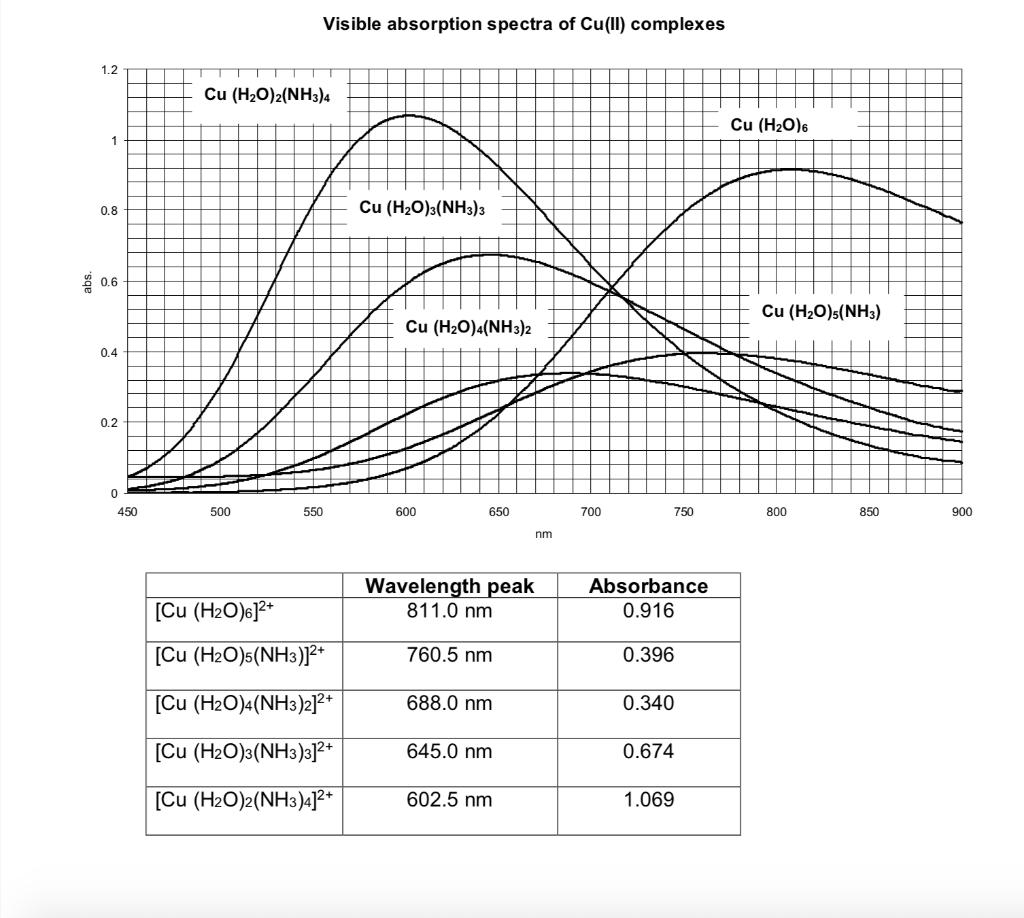
(i) Draw the electron distribution in the d orbitals of an octahedral Cu(II) complex and show the electronic transition responsible for the absorptions observed.
(ii) Assign the transition you observe for each complex and give the value of Δ (in cm-1).
1.2 1 0.8 0.6 0.4 0.2 0 450 Visible absorption spectra of Cu(II) complexes Cu (HO)2(NH3)4 500 550 [Cu (HO)6]+ [Cu (HO)5(NH3)]+ [Cu (H2O)4(NH3)2]+ [Cu (H2O)3(NH3)3]+ [Cu (H2O)2(NH3)4]+ Cu (HO)3(NH3)3 Cu (HO)4(NH3)2 600 650 Wavelength peak 811.0 nm 760.5 nm 688.0 nm 645.0 nm nm 602.5 nm 700 Absorbance 0.916 0.396 750 0.340 0.674 1.069 Cu (HO)6 Cu (HO)5(NH3) 800 850 900
Step by Step Solution
★★★★★
3.52 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
t The electronic distribution of Cu II complex having octahedad geometry is 2 Cu 18 Ar g3d9 1 0 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


