Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The elementary liquid phase reaction A+B C (k=0.01 L/mol/s at 27 C and Ea=10,000 cal/mol) is carried out in an adiabatic CSTR. An equal
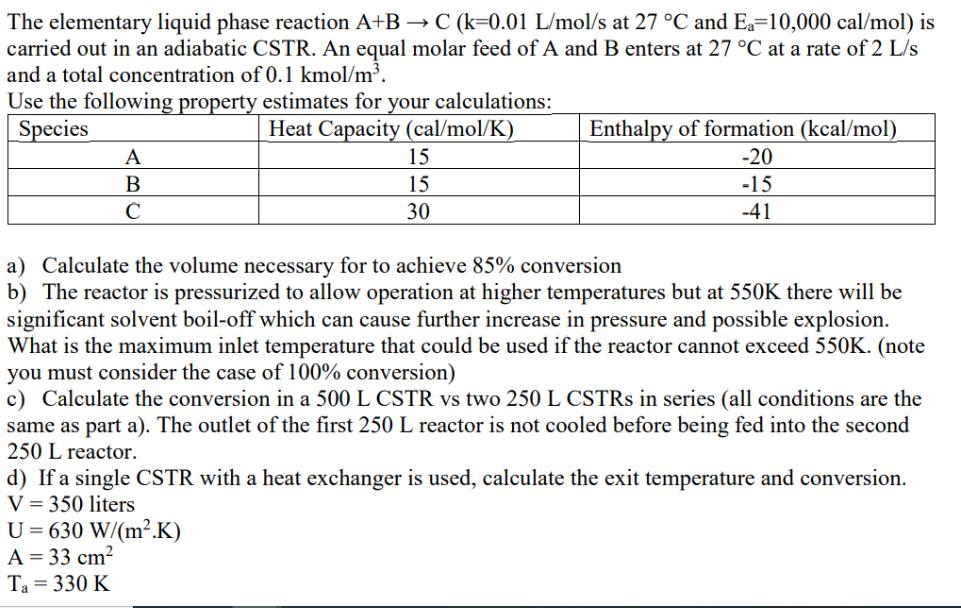
The elementary liquid phase reaction A+B C (k=0.01 L/mol/s at 27 C and Ea=10,000 cal/mol) is carried out in an adiabatic CSTR. An equal molar feed of A and B enters at 27 C at a rate of 2 L/s and a total concentration of 0.1 kmol/m. Use the following property estimates for your calculations: Species A B C Heat Capacity (cal/mol/K) 15 15 30 Enthalpy of formation (kcal/mol) -20 -15 a) Calculate the volume necessary for to achieve 85% conversion -41 b) The reactor is pressurized to allow operation at higher temperatures but at 550K there will be significant solvent boil-off which can cause further increase in pressure and possible explosion. What is the maximum inlet temperature that could be used if the reactor cannot exceed 550K. (note you must consider the case of 100% conversion) c) Calculate the conversion in a 500 L CSTR vs two 250 L CSTRS in series (all conditions are the same as part a). The outlet of the first 250 L reactor is not cooled before being fed into the second 250 L reactor. d) If a single CSTR with a heat exchanger is used, calculate the exit temperature and conversion. V = 350 liters U 630 W/(m2.K) A = 33 cm Ta=330 K
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


