Question
The elementary liquid-phase reaction: A + B C is to be carried out in a CSTR with three impellers. The mixing pattern in the
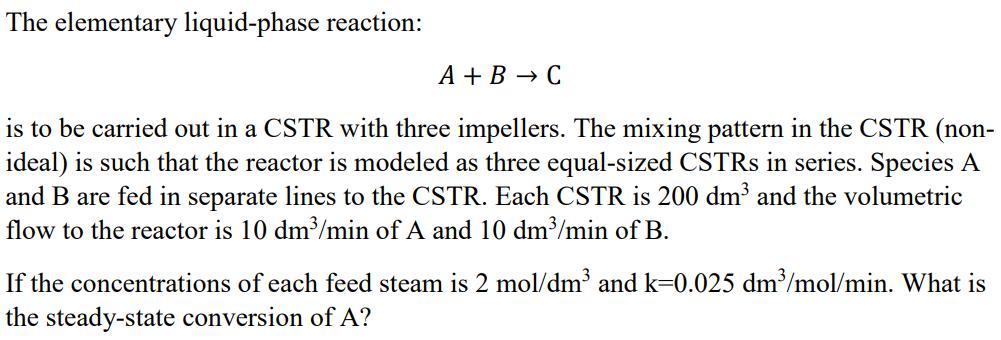
The elementary liquid-phase reaction: A + B C is to be carried out in a CSTR with three impellers. The mixing pattern in the CSTR (non- ideal) is such that the reactor is modeled as three equal-sized CSTRs in series. Species A and B are fed in separate lines to the CSTR. Each CSTR is 200 dm and the volumetric flow to the reactor is 10 dm/min of A and 10 dm/min of B. If the concentrations of each feed steam is 2 mol/dm and k=0.025 dm/mol/min. What is the steady-state conversion of A?
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To find the steadystate conversion of A in the CSTR system we need to analyze the reaction kinetics and the flow rates in each CSTR Given The CSTR sys...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



