Answered step by step
Verified Expert Solution
Question
1 Approved Answer
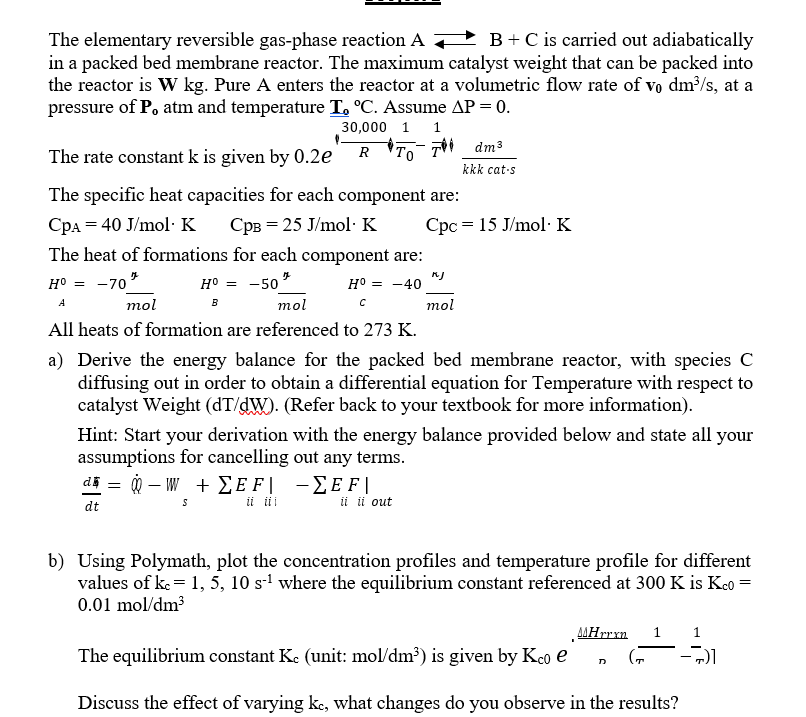
The elementary reversible gas - phase reaction A B + C is carried out adiabatically in a packed bed membrane reactor. The maximum catalyst weight
The elementary reversible gasphase reaction is carried out adiabatically
in a packed bed membrane reactor. The maximum catalyst weight that can be packed into
the reactor is Pure A enters the reactor at a volumetric flow rate of at a
pressure of atm and temperature Assume
The rate constant is given by
The specific heat capacities for each component are:
The heat of formations for each component are:
All heats of formation are referenced to
a Derive the energy balance for the packed bed membrane reactor, with species
diffusing out in order to obtain a differential equation for Temperature with respect to
catalyst Weight Refer back to your textbook for more information
Hint: Start your derivation with the energy balance provided below and state all your
assumptions for cancelling out any terms.
b Using Polymath, plot the concentration profiles and temperature profile for different
values of where the equilibrium constant referenced at is
Discuss the effect of varying what changes do you observe in the results?
W kg v dms Po atm To C
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


