Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The enthalpy for ATP hydrolysis is -24 kJ/mol at 37 C. The entropy for ATP hydrolysis 22 J/molk at 37 C. The free energy for


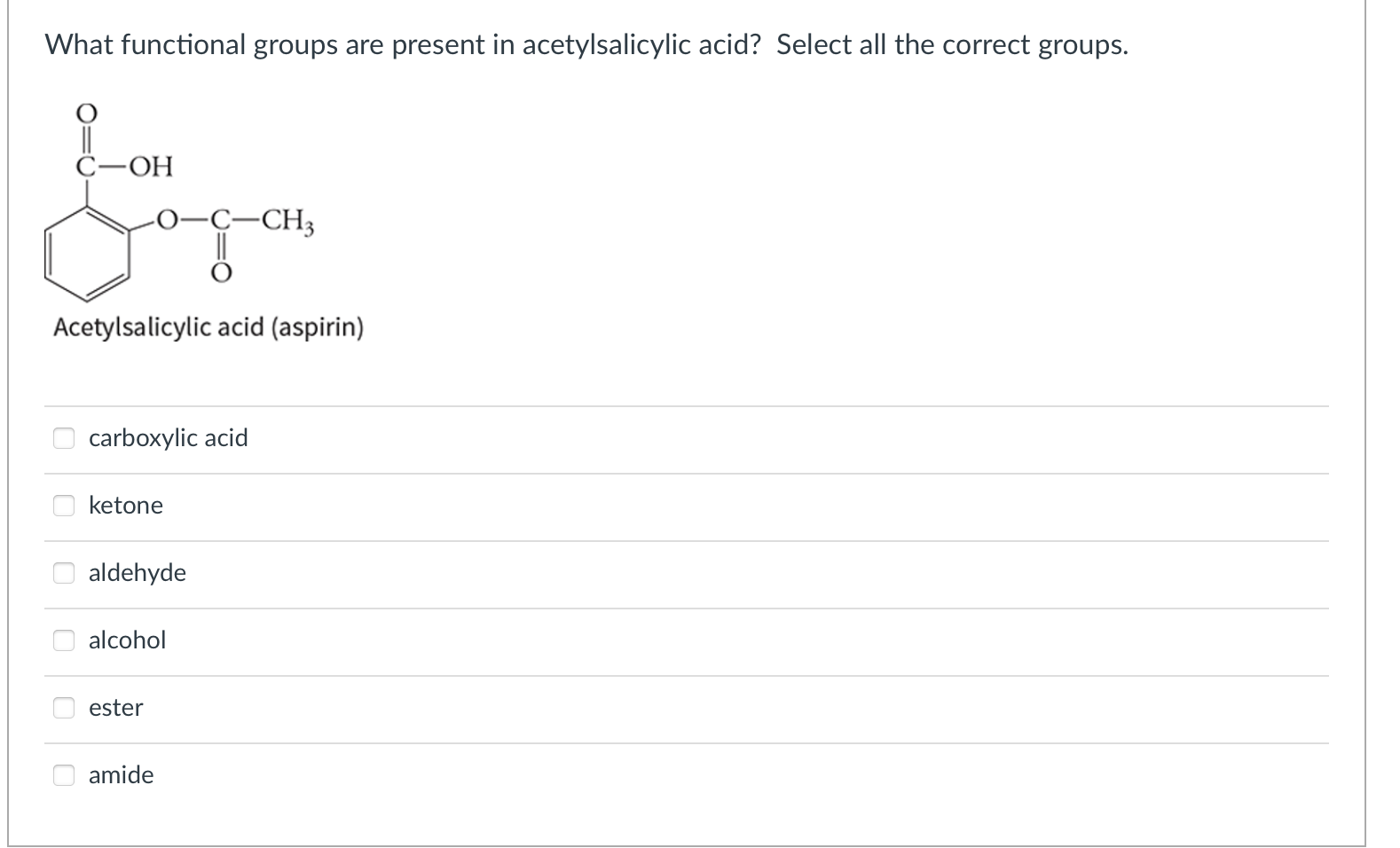
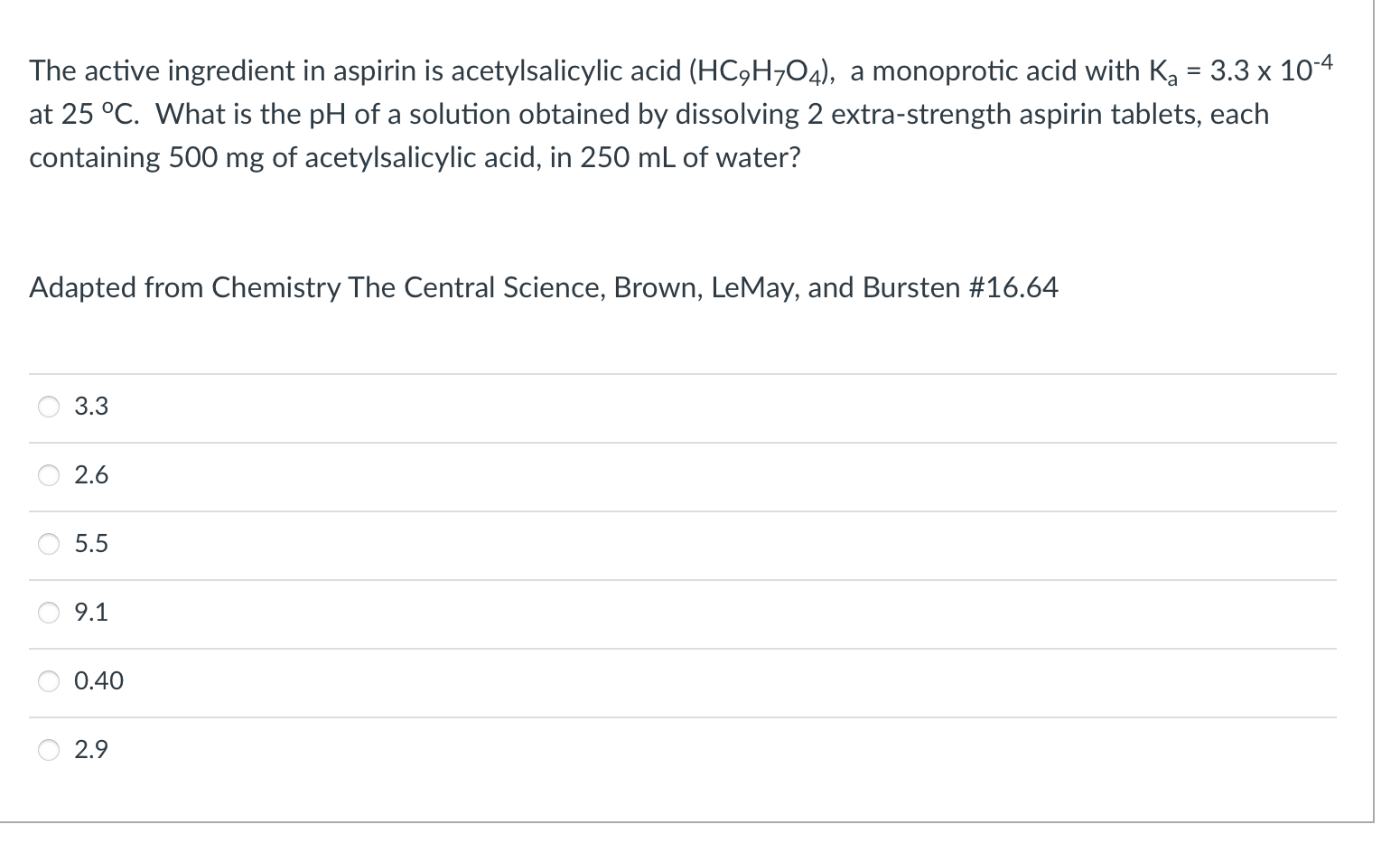
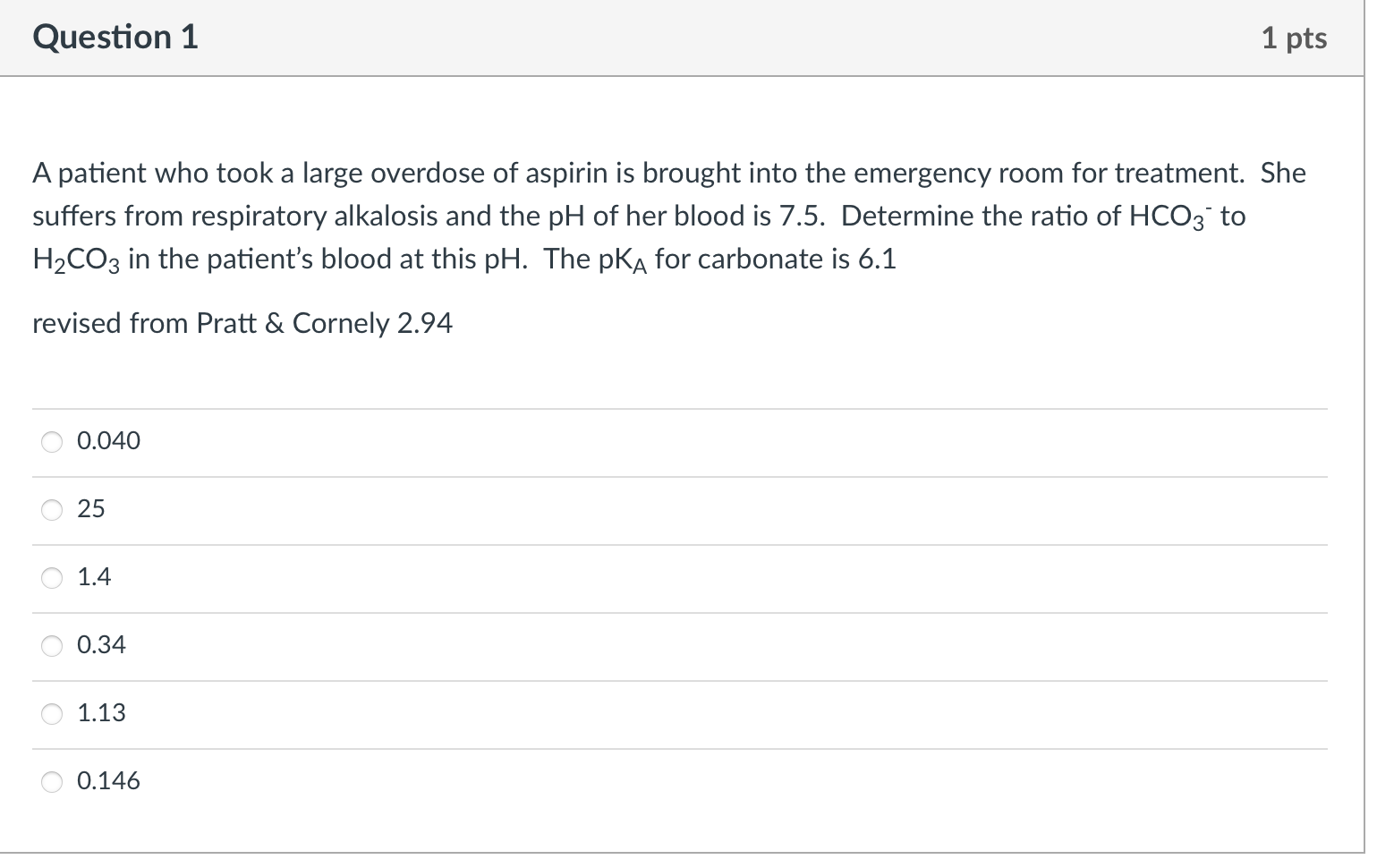
The enthalpy for ATP hydrolysis is -24 kJ/mol at 37 C. The entropy for ATP hydrolysis 22 J/molk at 37 C. The free energy for the conversion of glucose to glucose-6-phosphate is 17 kJ/mol at 37 C. What is the total free energy of the coupled reaction of glucose and ATP to form glucose-6-phosphate, ADP, phosphate, and water at 37 C? -31 kJ/mol -7 kJ/mol + 7 kJ/mol -14 kJ/mol +15 kJ/mol -48 kJ/mol The pKa of aspirin is 3.5. The pH is different in different places in the digestive system. In which conditions will the aspirin be protonated? saliva in mouth pH ~6.7 stomach pH ~2 ileum ~7.4 caecum ~5.7 rectum ~6.7 saliva stomach ileum caecum rectum What functional groups are present in acetylsalicylic acid? Select all the correct groups. C-OH -CH3 Acetylsalicylic acid (aspirin) carboxylic acid ketone aldehyde alcohol ester amide = The active ingredient in aspirin is acetylsalicylic acid (HC9H704), a monoprotic acid with Ka = 3.3 x 10-4 at 25 C. What is the pH of a solution obtained by dissolving 2 extra-strength aspirin tablets, each containing 500 mg of acetylsalicylic acid, in 250 mL of water? Adapted from Chemistry The Central Science, Brown, LeMay, and Bursten #16.64 3.3 2.6 5.5 9.1 5 0.40 2.9 Question 1 1 pts A patient who took a large overdose of aspirin is brought into the emergency room for treatment. She suffers from respiratory alkalosis and the pH of her blood is 7.5. Determine the ratio of HCO3 to H2CO3 in the patient's blood at this pH. The pka for carbonate is 6.1 revised from Pratt & Cornely 2.94 0.040 25 1.4 0.34 1.13 0.146
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


