Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The equation connecting the equilibrium constant of the ammonia formation reaction to temperature is as follows. Here T is the temperature as K and KT
The equation connecting the equilibrium constant of the ammonia formation reaction to temperature is as follows. Here T is the temperature as K and KT is the equilibrium constant. Accordingly, calculate the temperature at which KT is 0.0036 using the partial substitution method, which is one of the simple iteration methods (To= 273 K, approximation error = 0.01 and the partiality coefficient should be taken as r=0.7).
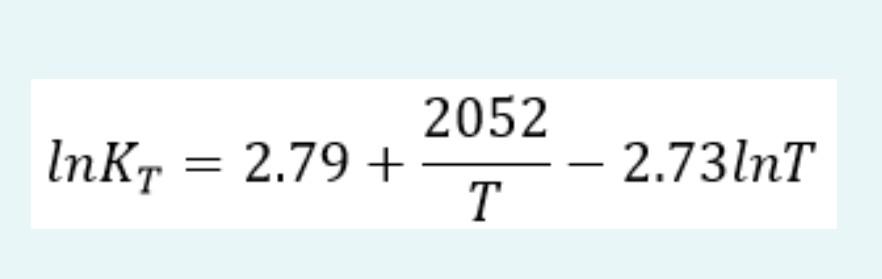
lnKT=2.79+T20522.73lnT
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


