Question
The equilibrium data for the extraction of acetic acid (A) from water (D) to isopropyl ether (S) are given in table 14-2. there is 1000
The equilibrium data for the extraction of acetic acid (A) from water (D) to isopropyl ether (S) are given in table 14-2. there is 1000 kg/h of a feed containing 40% A and 60% D, by weight. this feed is extracted with a total of 1000 kg/h of pure isopropyl ether in a two-stage system.
a. If a cross-flow system is used with 500 kg/h of isopropyl ether to each stage, calculate the compositions of the extracts, the final refining composition, and the flow rate of the refining leaving the system.
b. If a two-stage countercurrent system is used, with a solvent flow rate of 1000 kg/h, calculate the extract composition, the refining composition, and the refining flow rate. If the difference point goes off the page, attach another sheet of paper to locate it.
c. Compare the crossflow and counterflow systems.
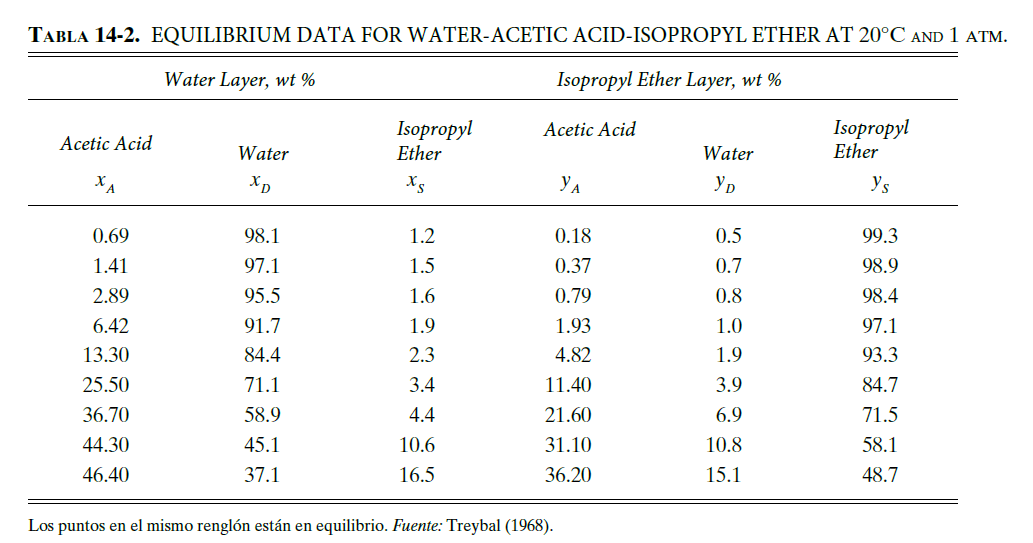
Wankat 2 edition. Liquid liquid extraction with cross flow and countercurrent flow
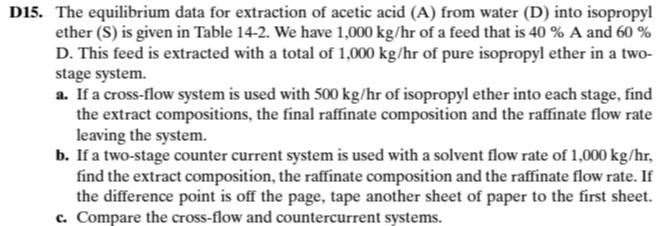
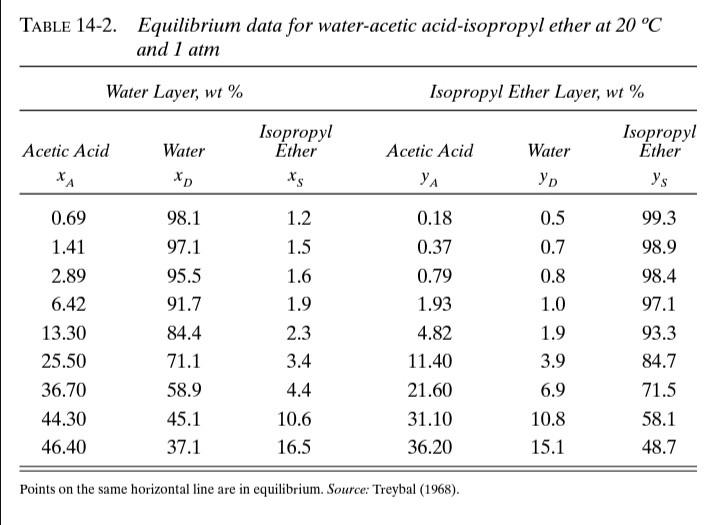
TABLA 14-2. EQUILIBRIUM DATA FOR WATER-ACETIC ACID-ISOPROPYL ETHER AT 20C AND 1 ATM. Water Layer, wt % Isopropyl Ether Layer, wt % Acetic Acid Acetic Acid Water Isopropyl Ether Xs Water Isopropyl Ether ys YA 0.69 99.3 1.41 2.89 6.42 13.30 25.50 36.70 44.30 46.40 98.1 97.1 95.5 91.7 84.4 71.1 58.9 45.1 37.1 1.2 1.5 1.6 1.9 2.3 3.4 4.4 0.18 0.37 0.79 1.93 4.82 11.40 0.5 0.7 0.8 1.0 1.9 3.9 6.9 10.8 15.1 98.9 98.4 97.1 93.3 84.7 71.5 58.1 48.7 21.60 10.6 31.10 36.20 16.5 Los puntos en el mismo rengln estn en equilibrio. Fuente: Treybal (1968). D15. The equilibrium data for extraction of acetic acid (A) from water (D) into isopropyl ether (S) is given in Table 14-2. We have 1,000 kg/hr of a feed that is 40 % A and 60 % D. This feed is extracted with a total of 1,000 kg/hr of pure isopropyl ether in a two- stage system. a. If a cross-flow system is used with 500 kg/hr of isopropyl ether into each stage, find the extract compositions, the final raffinate composition and the raffinate flow rate leaving the system. b. If a two-stage counter current system is used with a solvent flow rate of 1,000 kg/hr, find the extract composition, the raffinate composition and the raffinate flow rate. If the difference point is off the page, tape another sheet of paper to the first sheet. c. Compare the cross-flow and countercurrent systems. TABLE 14-2. Equilibrium data for water-acetic acid-isopropyl ether at 20 C and 1 atm Water Layer, wt% Isopropyl Ether Layer, wt% Acetic Acid Water Isopropyl Ether xs Acetic Acid YA Water Isopropyl Ether y's *A 99.3 0.69 1.41 2.89 6.42 13.30 25.50 36.70 44.30 46.40 98.1 97.1 95.5 91.7 84.4 71.1 58.9 45.1 37.1 1.2 1.5 1.6 1.9 2.3 3.4 4.4 10.6 16.5 0.18 0.37 0.79 1.93 4.82 11.40 21.60 31.10 36.20 0.5 0.7 0.8 1.0 1.9 3.9 6.9 10.8 15.1 98.9 98.4 97.1 93.3 84.7 71.5 58.1 48.7 Points on the same horizontal line are in equilibrium. Source: Treybal (1968)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


