Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The evaporator section of a heat pump is installed in a large tank of water, which is used as a heat source during the
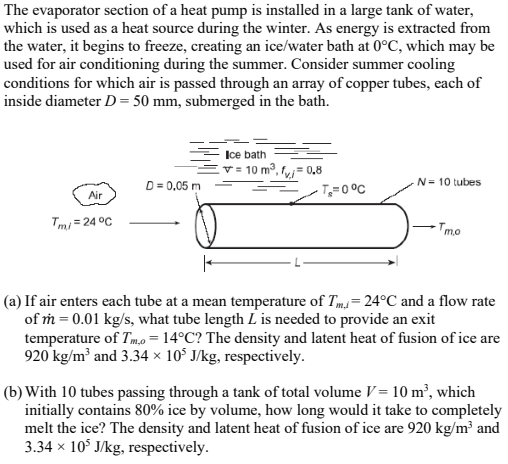

The evaporator section of a heat pump is installed in a large tank of water, which is used as a heat source during the winter. As energy is extracted from the water, it begins to freeze, creating an ice/water bath at 0C, which may be used for air conditioning during the summer. Consider summer cooling conditions for which air is passed through an array of copper tubes, each of inside diameter D = 50 mm, submerged in the bath. Ice bath 10 m, fv=0.8 D= 0,05 m N= 10 tubes T=0C Air Tmi 24C Tmo (a) If air enters each tube at a mean temperature of T = 24C and a flow rate of m = 0.01 kg/s, what tube length L is needed to provide an exit temperature of Tm,o=14C? The density and latent heat of fusion of ice are 920 kg/m and 3.34 105 J/kg, respectively. (b) With 10 tubes passing through a tank of total volume V = 10 m, which initially contains 80% ice by volume, how long would it take to completely melt the ice? The density and latent heat of fusion of ice are 920 kg/m and 3.34 105 J/kg, respectively. (c) The air outlet temperature may be regulated by adjusting the tube mass flow rate. For the tube length determined in part (a), compute and plot Tm,o as a function of m for 0.005 m0.05 kg/s.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


