Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The exothermic reaction of stilbene (A) to form the economically important trospophene (B) and methane (C), i.e., AB+C was carried out isothermally, and the following
The exothermic reaction of stilbene (A) to form the economically important trospophene (B) and methane (C), i.e., AB+C was carried out isothermally, and the following data were recorded:
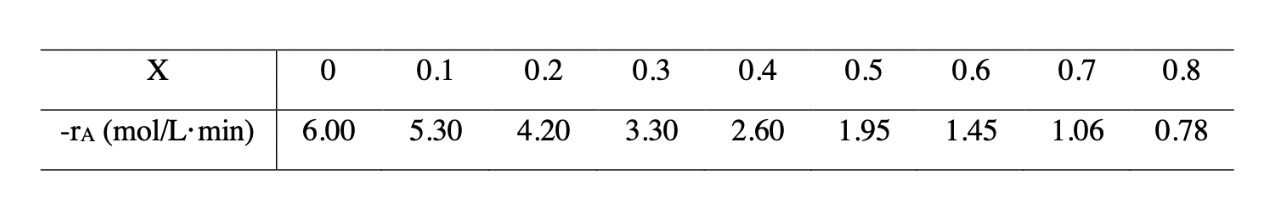 The entering molar flow rate of A was 300 mol/min.
The entering molar flow rate of A was 300 mol/min.
(a) What is the conversion that can be achieved in a 100-L CSTR? (b) What is the conversion that can be achieved in a 100-L PFR? (c) What conversion can be achieved if a 27-L CSTR is followed in series by a 42-L PFR? (d) What conversion can be achieved if a 27-L PFR is followed in series by a 42-L CSTR?
\begin{tabular}{c|ccccccccc} \hline X & 0 & 0.1 & 0.2 & 0.3 & 0.4 & 0.5 & 0.6 & 0.7 & 0.8 \\ \hline -rA (mol/Lmin) & 6.00 & 5.30 & 4.20 & 3.30 & 2.60 & 1.95 & 1.45 & 1.06 & 0.78 \\ \hline \end{tabular}Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


