Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The feed (F) to a separation process enters at 200 mol/min and contains 40 mol% ethanol and the remainder water. The separator can only
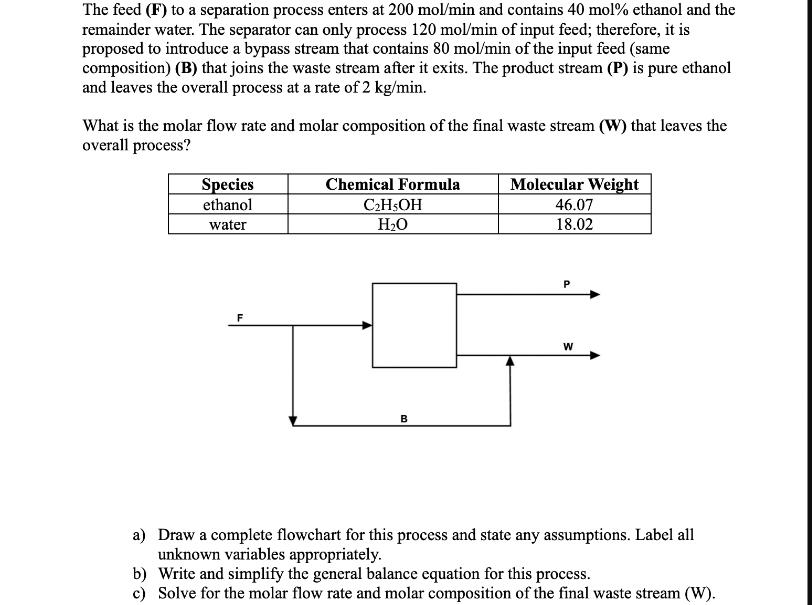
The feed (F) to a separation process enters at 200 mol/min and contains 40 mol% ethanol and the remainder water. The separator can only process 120 mol/min of input feed; therefore, it is proposed to introduce a bypass stream that contains 80 mol/min of the input feed (same composition) (B) that joins the waste stream after it exits. The product stream (P) is pure ethanol and leaves the overall process at a rate of 2 kg/min. What is the molar flow rate and molar composition of the final waste stream (W) that leaves the overall process? Species ethanol water F Chemical Formula CH5OH HO B Molecular Weight 46.07 18.02 P W a) Draw a complete flowchart for this process and state any assumptions. Label all unknown variables appropriately. b) Write and simplify the general balance equation for this process. c) Solve for the molar flow rate and molar composition of the final waste stream (W).
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Lets tackle the questions one by one a Flowchart Assumptions Steadystate operation Negligible heat a...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


