Question
The fermentation bioreactor enables cell growth to take place under well-defined operating conditions. Below is a typical overall fermentation reaction: 100 Media + 70 Oxygen
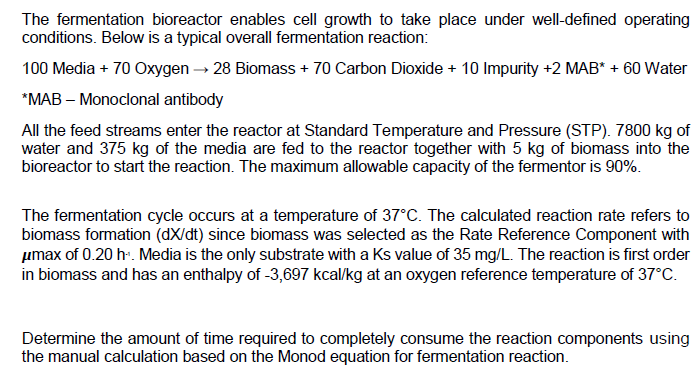
The fermentation bioreactor enables cell growth to take place under well-defined operating conditions. Below is a typical overall fermentation reaction:
100 Media + 70 Oxygen 28 Biomass + 70 Carbon Dioxide + 10 Impurity +2 MAB* + 60 Water
*MAB Monoclonal antibody
All the feed streams enter the reactor at Standard Temperature and Pressure (STP). 7800 kg of water and 375 kg of the media are fed to the reactor together with 5 kg of biomass into the bioreactor to start the reaction. The maximum allowable capacity of the fermentor is 90%.
The fermentation cycle occurs at a temperature of 37C. The calculated reaction rate refers to biomass formation (dX/dt) since biomass was selected as the Rate Reference Component with max of 0.20 h-1. Media is the only substrate with a Ks value of 35 mg/L. The reaction is first order in biomass and has an enthalpy of -3,697 kcal/kg at an oxygen reference temperature of 37C.
Determine the amount of time required to completely consume the reaction components using the manual calculation based on the Monod equation for fermentation reaction.
The fermentation bioreactor enables cell growth to take place under well-defined operating conditions. Below is a typical overall fermentation reaction: 100 Media + 70 Oxygen 28 Biomass + 70 Carbon Dioxide + 10 Impurity +2 MAB* + 60 Water *MAB - Monoclonal antibody All the feed streams enter the reactor at Standard Temperature and Pressure (STP). 7800 kg of water and 375 kg of the media are fed to the reactor together with 5 kg of biomass into the bioreactor to start the reaction. The maximum allowable capacity of the fermentor is 90%. The fermentation cycle occurs at a temperature of 37C. The calculated reaction rate refers to biomass formation (dx/dt) since biomass was selected as the Rate Reference Component with umax of 0.20 h.. Media is the only substrate with a Ks value of 35 mg/L. The reaction is first order in biomass and has an enthalpy of -3,697 kcal/kg at an oxygen reference temperature of 37C. Determine the amount of time required to completely consume the reaction components using the manual calculation based on the Monod equation for fermentation reactionStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


