Question
The figure below shows the zinc blende unit cell (left) and actual ion packing (right). O Zn Two ions per latice point Structure: Zinc
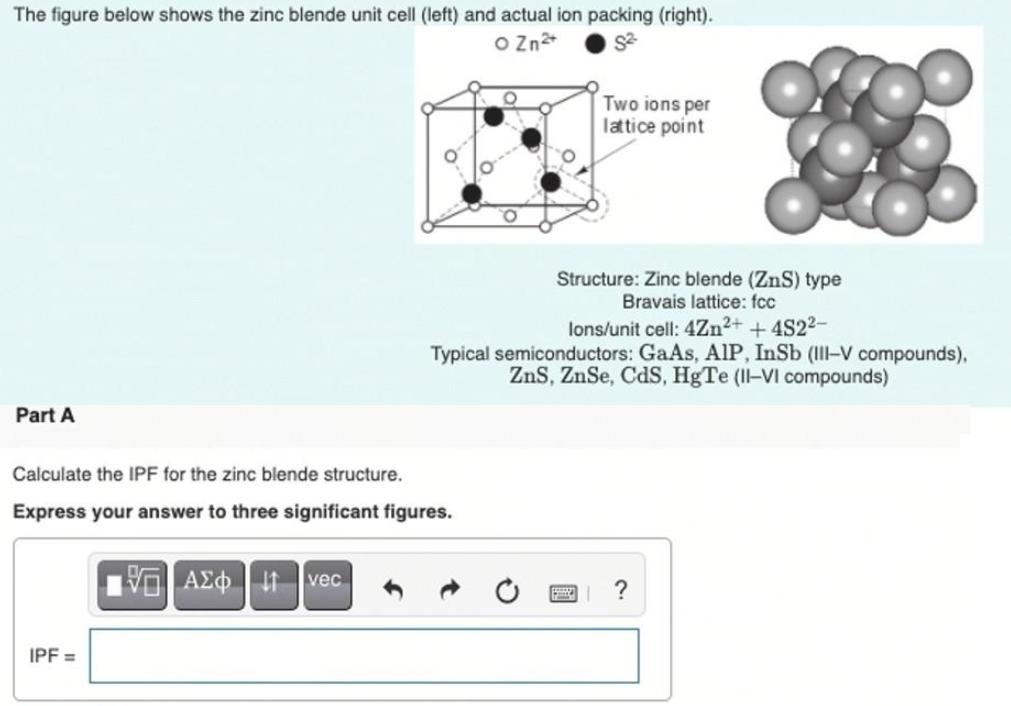
The figure below shows the zinc blende unit cell (left) and actual ion packing (right). O Zn Two ions per latice point Structure: Zinc blende (ZnS) type Bravais lattice: fcc lons/unit cell: 4Zn2+ +4S22- Typical semiconductors: GaAs, AlP, InSb (III-V compounds), ZnS, ZnSe, CdS, HgTe (II-VI compounds) Part A Calculate the IPF for the zinc blende structure. Express your answer to three significant figures. vec IPF =
Step by Step Solution
3.34 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
Answer Solution Ch zinc Blende tnit cell at Cakon Ahian E...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Core Macroeconomics
Authors: Eric Chiang
3rd edition
978-1429278478, 1429278471, 978-1429278492, 1429278498, 1464191433, 978-1464191435
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



