Question
The figure (not to scale) shows a pV diagram for 3.5 g of helium gas (He) that undergoes the process 1 2 3. The

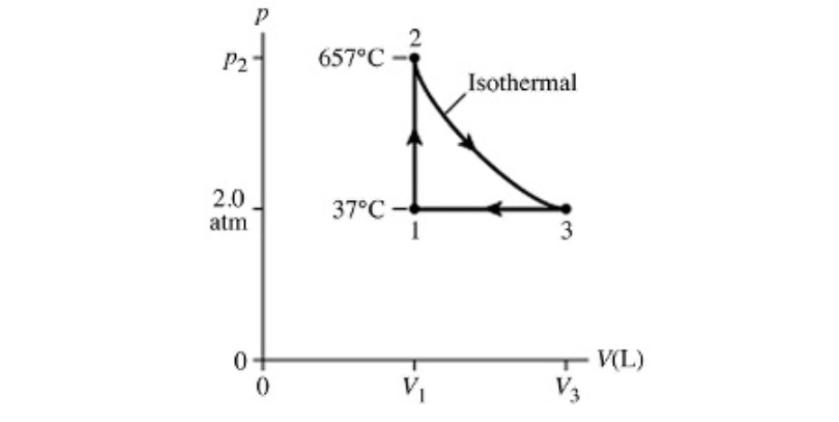
The figure (not to scale) shows a pV diagram for 3.5 g of helium gas (He) that undergoes the process 1 2 3. The ideal gas constant is R = 8.314 J/mol K = 0.0821 L atm/mol K, and the atomic weight of helium is 4.0 g/mol. What is the value of V3 in liters? Please give your numerical answer with one decimal place. . . P 2 P2 657C Isothermal 2.0 37C atm 1 3 0. V(L) 0 V V3
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics for Scientists and Engineers A Strategic Approach with Modern Physics
Authors: Randall D. Knight
4th edition
978-0134092508, 134092503, 133942651, 978-0133942651
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



