Question
The figure shows the electron energy-level diagram during a collision between a helium atom and a neon atom, which occurs in helium-neon (HeNe) lasers. A
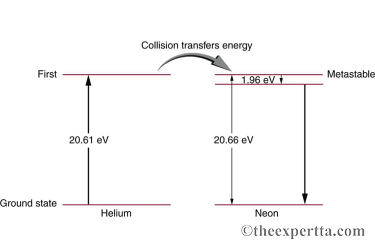
The figure shows the electron energy-level diagram during a collision between a helium atom and a neon atom, which occurs in helium-neon (HeNe) lasers. A population of electrons is created in the metastable state of the neon atoms via collisions with excited helium atoms. This excess of electrons in an excited state is called "population inversion".
A) What is the energy, in electron volts, of the photon emitted when an electron in neon goes from its metastable state to the one immediately below?
B) What is the wavelength of this radiation in nanometers?
C) What wavelength, in nanometers, is emitted when the electron in neon makes a direct transition, from the metastable state, to its ground state?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


