Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The flask shown here contains 10.0 mL of HCI and a few drops of phenolphthalein indicator. The buret contains 0.290 M NAH. mL Reset
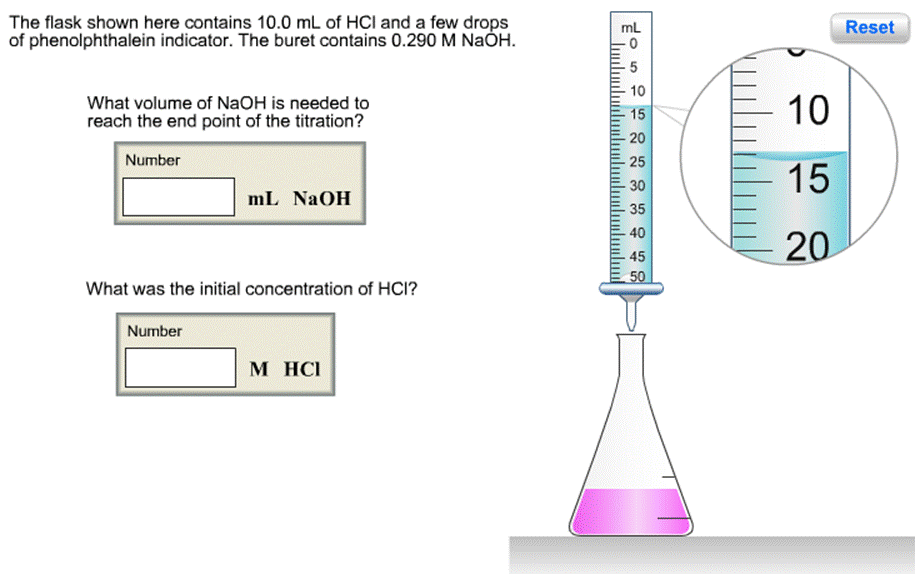
The flask shown here contains 10.0 mL of HCI and a few drops of phenolphthalein indicator. The buret contains 0.290 M NAH. mL Reset 10 What volume of NaOH is needed to reach the end point of the titration? 10 15 20 Number 25 15 30 mL NAOH 35 40 20 45 50 What was the initial concentration of HCI? Number M HCI Click to begin The flask shown here contains 10.0 mL of HCI and a few drops of phenolphthalein indicator. The buret contains 0.290 M NAH. mL Reset 10 What volume of NaOH is needed to reach the end point of the titration? 10 15 20 Number 25 15 30 mL NAOH 35 40 20 45 50 What was the initial concentration of HCI? Number M HCI Click to begin The flask shown here contains 10.0 mL of HCI and a few drops of phenolphthalein indicator. The buret contains 0.290 M NAH. mL Reset 10 What volume of NaOH is needed to reach the end point of the titration? 10 15 20 Number 25 15 30 mL NAOH 35 40 20 45 50 What was the initial concentration of HCI? Number M HCI Click to begin
Step by Step Solution
★★★★★
3.45 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
15 griuen thed Velume of Uve lom Volume of Ma on V6 Conc...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


