Answered step by step
Verified Expert Solution
Question
1 Approved Answer
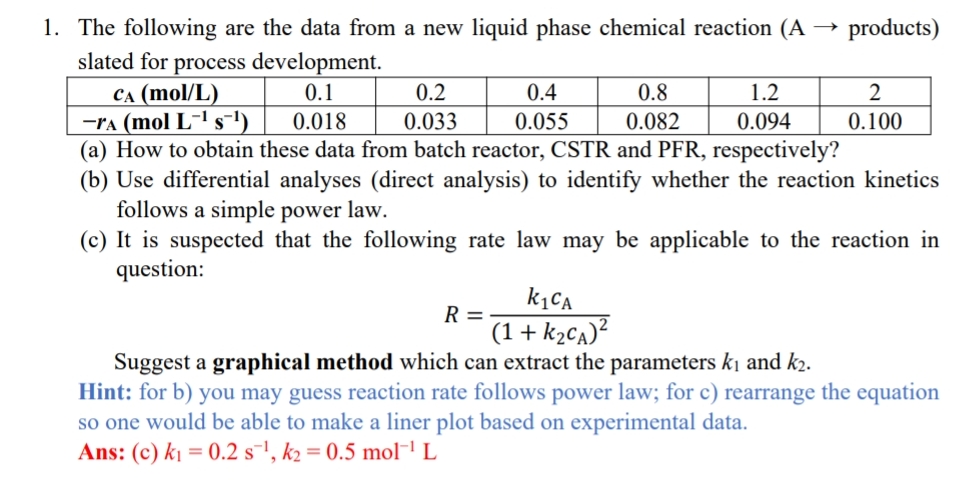
The following are the data from a new liquid phase chemical reaction products ) slated for process development. table [ [ c A (
The following are the data from a new liquid phase chemical reaction products slated for process development.
table
a How to obtain these data from batch reactor, CSTR and PFR respectively?
b Use differential analyses direct analysis to identify whether the reaction kinetics follows a simple power law.
c It is suspected that the following rate law may be applicable to the reaction in question:
Suggest a graphical method which can extract the parameters and
Hint: for b you may guess reaction rate follows power law; for c rearrange the equation so one would be able to make a liner plot based on experimental data.
Ans: c
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


