Answered step by step
Verified Expert Solution
Question
1 Approved Answer
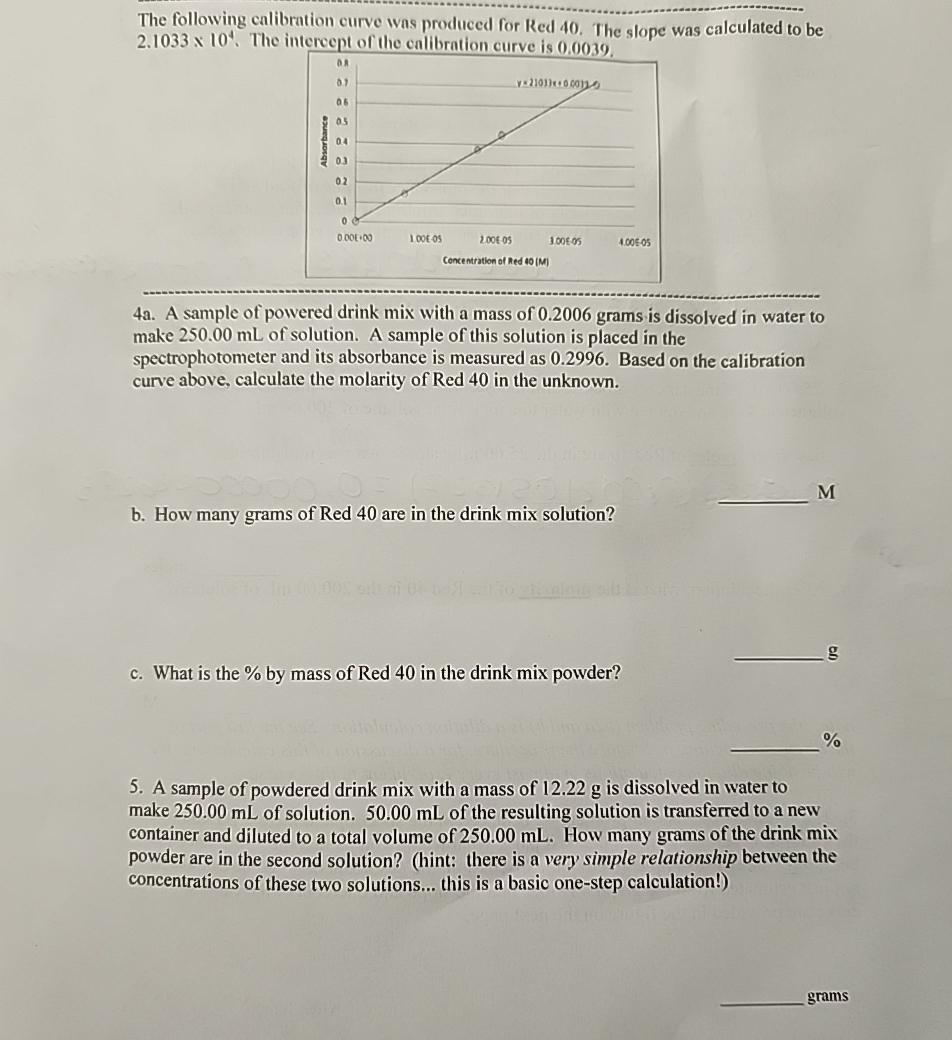
The following calibration curve was produced for Red 4 0 . The slope was calculated to be 2 . 1 0 3 3 1 0
The following calibration curve was produced for Red The slope was calculated to be The
a A sample of powered drink mix with a mass of grams is dissolved in water to make of solution. A sample of this solution is placed in the spectrophotometer and its absorbance is measured as Based on the calibration curve above, calculate the molarity of Red in the unknown.
b How many grams of Red are in the drink mix solution?
c What is the by mass of Red in the drink mix powder?
A sample of powdered drink mix with a mass of is dissolved in water to make of solution. of the resulting solution is transferred to a new container and diluted to a total volume of How many grams of the drink mix powder are in the second solution? hint: there is a very simple relationship between the concentrations of these two solutions... this is a basic onestep calculation!
grams
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


