Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The following data in the attached spreadsheet were obtained for an irreversible elementary reaction. Plot the linearized form of the integrated rate equations (Refer to
The following data in the attached spreadsheet were obtained for an irreversible elementary reaction. Plot the linearized form of the integrated rate equations (Refer to Table 5-5 in your textbook) for a zero, first, and second order reaction. (You should have three separate graphs.) Determine the order of the reaction and the rate constant k by doing the following:
1) Right click on the curve and click "Add Trendline." In the options that appear, click "Display Equation on Chart" and "Display R-squared value on chart." Do this for all three curves.
2) The R2 value that is closest to one corresponds to the curve that best fits the associated equation. (You should remember from statics that R2 is a measure of the best fit of a linear regression.) This curve is the equation corresponding to the reaction order of the data.
3) Once you have determined the order, use the equation shown on the curve and match the variables and values with the integrated equation. Determine the rate constant. (Hint: rate constants are NOT negative.)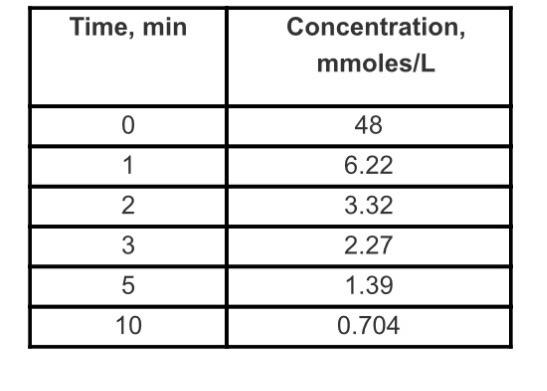
1) Right click on the curve and click "Add Trendline." In the options that appear, click "Display Equation on Chart" and "Display R-squared value on chart." Do this for all three curves.
2) The R2 value that is closest to one corresponds to the curve that best fits the associated equation. (You should remember from statics that R2 is a measure of the best fit of a linear regression.) This curve is the equation corresponding to the reaction order of the data.
3) Once you have determined the order, use the equation shown on the curve and match the variables and values with the integrated equation. Determine the rate constant. (Hint: rate constants are NOT negative.)

The order of reaction is:
The rate constant is . (Type only the value of the rate constant here rounded to four decimal places. On your paper, write the value with the correct units.)
blank1 - Numeric Answer
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


