Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The following data is available for a binary mixture of components (1) and (2) at T = 308.15 K: Saturation pressures of pure components:
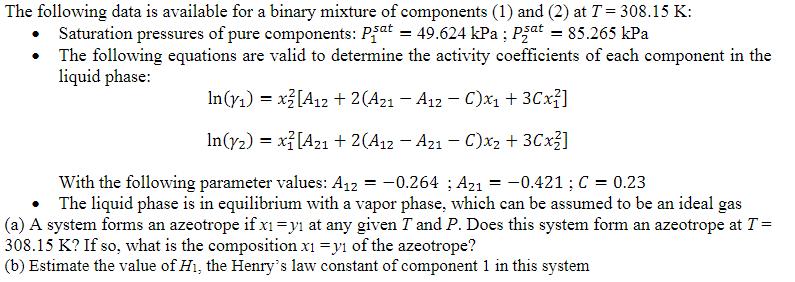
The following data is available for a binary mixture of components (1) and (2) at T = 308.15 K: Saturation pressures of pure components: Pat = 49.624 kPa ; Pat = 85.265 kPa The following equations are valid to determine the activity coefficients of each component in the liquid phase: In(y) = x2 [A12 + 2(A21 - A12 - C)x + 3Cx] In(y2) = x[A21 + 2(A12 - A21 - C)x + 3Cx] With the following parameter values: A12 = -0.264; A21 = -0.421 ; C = 0.23 The liquid phase is in equilibrium with a vapor phase, which can be assumed to be an ideal gas (a) A system forms an azeotrope if x1 = y at any given T and P. Does this system form an azeotrope at T = 308.15 K? If so, what is the composition x1 = y of the azeotrope? (b) Estimate the value of Hi, the Henry's law constant of component 1 in this system
Step by Step Solution
★★★★★
3.34 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
ylvan data Pin l A The G CUNG I 44624 kpG C264 A T t 3081151 Pint 85265 14 Pa 2 421 to de...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


