Question
The following data were obtained during the first order thermal decomposition of SOCl at a constant volume. CBSE DELHI 2014 SOCl (g) - -
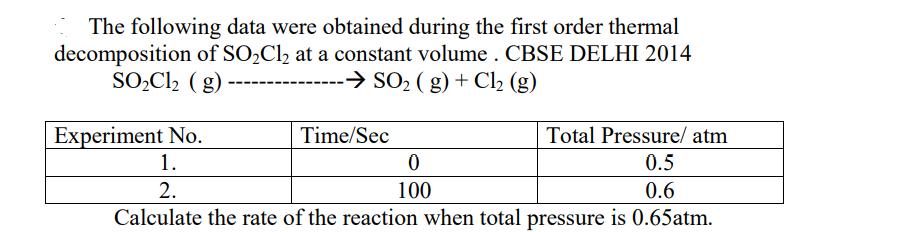
The following data were obtained during the first order thermal decomposition of SOCl at a constant volume. CBSE DELHI 2014 SOCl (g) - - SO (g) + Cl (g) Experiment No. Total Pressure/ atm 1. 0.5 2. 0.6 Calculate the rate of the reaction when total pressure is 0.65atm. Time/Sec 0 100
Step by Step Solution
3.38 Rating (142 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introduction To Probability And Statistics
Authors: William Mendenhall, Robert Beaver, Barbara Beaver
14th Edition
1133103758, 978-1133103752
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



