Question
The following emfs refer to the cell at 298 K: Pt | H(1 bar) | LiOH(0.01 mol dm3), LiCl(m) | AgCl(s) | Ag 0.01
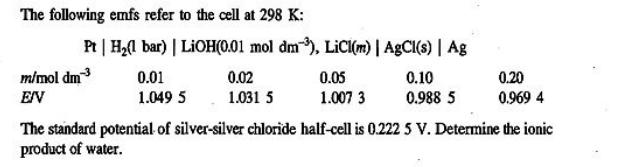
The following emfs refer to the cell at 298 K: Pt | H(1 bar) | LiOH(0.01 mol dm3), LiCl(m) | AgCl(s) | Ag 0.01 1.049 5 m/mol dm EIV 0.02 1.031 5 0.05 1.007 3 0.10 0.988 5 0.20 0.969 4 The standard potential of silver-silver chloride half-cell is 0.222 5 V. Determine the ionic product of water.
Step by Step Solution
3.45 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Precalculus
Authors: Michael Sullivan
9th edition
321716835, 321716833, 978-0321716835
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



