Question
1. The following enthalpy changes are known for reactions at 25 C and one atm. No Reaction AH (kJ/mol) CsHolg) + Hz{g) CHs(g) -123.8
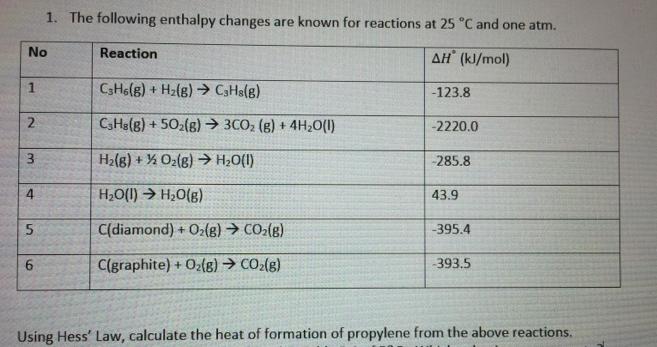
1. The following enthalpy changes are known for reactions at 25 "C and one atm. No Reaction AH (kJ/mol) CsHolg) + Hz{g) CHs(g) -123.8 2 CHa(g) + 502(8) 3CO; (8) + 4H20(1) -2220.0 3. Ha(8) + % Oz(8)20(1) -285.8 H20(1) H,0(g) 43.9 5. C(diamond) + O(g) CO2(g) -395.4 C(graphite) + 02(8) CO2(8) 393.5 Using Hess' Law, calculate the heat of formation of propylene from the above reactions.
Step by Step Solution
3.50 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
3Cgraphite 3H2g C3H6g multiply 1 with 3 3Cgraphite 3O2g 3CO2g ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Statistics Picturing The World
Authors: Ron Larson, Betsy Farber
7th Edition
134683412, 978-0134683416
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



