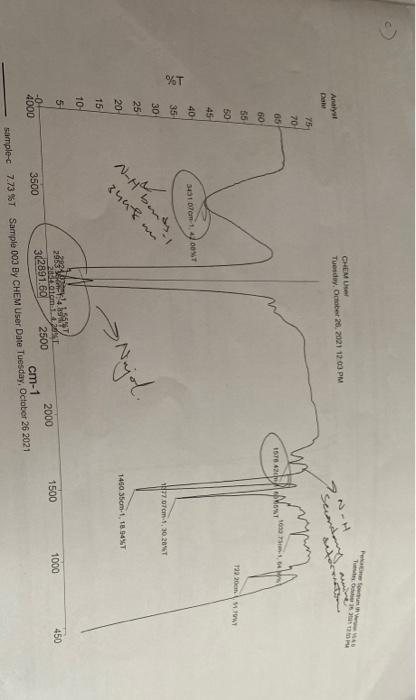
the following experiments, nickel complexes of two isomeric ligands derived from NMR, MS and IR spectral measurements can be used to investigate the structures of diaminopropane and Pyrrole-2-aldehyde are prepared using different methods. 'H these compounds Experimental a) A Schiff base ligand from 1,3-diaminopropane and pyrrole-2-aldehyde This reaction is to be carried out in the designated fume cupboard. Dissolve pyrrole-2- aldehyde (0.95 g) in ethanol (5 cm) in a round-bottomed "Quickfit flask (100 cm). a graduated pipette, add 1,3-diaminopropane (0.40 cm) to the solution and mix Using the liquids, Fit a reflux condenser and warm the flask and reflux for 3-4 min and then stand in an ice bath for 2 hours. The mixture may solidify to a crystalline massor remain liquid. If a solid has deposited on cooling, collect this by filtration and wash it with a few cm of cold BOH. The combined filtrate and washing may deposit more products, as crystalline needles, on standing. If the mixture remains liquid, reduce the volume on a rotary evaporator, until solid starts to appear and then stand the flaskin an ice bath to complete the crystallization. Proceed to collect the product as described above. Allow the product to air dry and record your yicid. Obtain the IR (KBr disc). 'H NMR and mass spectra of your product. b) A nickel(II) complex form the Schiff base ligand Dissolve a portion (0.5 g) of the ligand prepared above is a) in warm ethanol (10 em Slowly add a solution of nickel ethanoate (nickel acetate, Ni(OCOCH3)2-4H-0,0.58) in water (10 cm) to produce a turbid, brick red mixture. Next add a solution of sodium carbonate (0.2 g) in water (5 cm) and stir the mixture for 20 minutes. After this time, collect the crude product by filtration and wash it with a little ethanol-water mixture (1:1, a few cm). Redissolve the red product in dichloromethane (ca. 40 cm) and dry the solution over a linic anhydrous magnesium sulfate. Remove the magnesium sulfate by filtration and wash it with a little dichloromethane. Add light- petroleum (80-100C, 40 cm) to the combined washings and filtrate, then remove the dichloromethane using a rotary evaporator (use a room temperature water bath, do not heat the flask). The red product will precipitate from the light petroleum as the dichloromethane is removed. The product may be collected by filtration and air dried. Record your yield and obtain the IR (KBr disc), 'H NMR and mass spectra of your product cm OR c) A nickel(II) complex with 1,2-diaminopropane and pyrrole-2-aldehyde equipped with a twin-necked adaptor, reflux condenser and dropping funnel. Place In the designated fume cupboard, set up a round-bottomed "Quickfit" flask (100 ethanol-water mixture (1:1 w/w. 50 cm) in the flask along with pyrrole-2-aldehyde anti-bumping granules. Heat the flask to dissolve the nickel ethanoate (a turbid rather (0.95 g), nickel ethanoate (nickel acetate, Ni(OCOCH3)2-4H20, 1.25 g) and 3 than clear solution will form) and then add an aqueous solution of NaOH (10% wiv, refluxing suspension of nickel hydroxide and aldehyde. Next, add water (10 cm) Add this diamine solution dropwise, over a period of about 20 minutes, love cm). Dissolve 1,2-diaminopropane (0.4 cm') in water (20 cm') in the dropping fuel with a little ethanol-water (1:1). Redissolve the product in dichloromethane (ca. 4) allow the mixture to cool. Collect the crude orange product by filtration and wash cm') while still in the filter funnel and allow the orange dichloromethane solution magnesium sulfate with a little dichloromethane. Add light-petroleum (80-100C) to magnesium sulfate, remove the magnesium sulfate by filtration and wash the filter into a clean conical flask (100 cm'). Dry this solution with a little anhydrous the combined filtrate and washings, and remove the dichloromethane using a rovary evaporator. (Use a room temperature water bath and do not heat the flask). The orange product will precipitate from the light-petroleum as the dichloromethane is removed and your yield may then be collected by filtration and allowed to dry in air. Record and obtain the IR (KBr disc), 'H NMR and mass spectra of your product. wo - Analyst Dale CHEMU Tuesday, October 26, 2021 19:03 PM Secondary 75 70 un 05 M. 1578AOSAT 60 55 50 1922 1941 45 40 301 7cm 1.4 T %T 35 N77 cm.1, 30.204T 30 25 20 N-H berts, 1450 35cm, 18.54%T qual > Najol. 15 TO 450 1000 1500 5 28833454 VAN -0- 2010 4000 3500 302891.60 2500 2000 cm-1 sample 7.73 %T Sample 003 BY CHEM User Date Tuesday, October 26 2021 CHEMU Tuesday, October 26, 2021 11:50 AM Anh Date 44 40 38 2. GAN 23 NO.254 1491 10,355 150,32225 1441 m2 tons SANT BY OST 36 34 32 3040m 1 an. 30 28 26 24 22 2 2043 1114 1145T 300 T2850 200LT 101 m: 1.27 14213-1.27 239 To stom-1.25 67% %T 254 25543T Buuhan A- bend CEN 77 band 1638mm 20 18 1000 450 16 14 12 16376cm-1, 11.51%T 11 4000 3500 362891.60 2500 2000 1500 cm-1 CHEM User 02_1 34 21 %T Sample 002 By CHEM User Date Tuesday, October 26 2021 How to CHEMU Tedy 2021150 PM Analys Date 54 50 YMT 45 1015 m., 1925T 40 35 11.30 4T 30 %T 1.1.2454 25 20 3436cm N-H band 15 Lords 1594am 10 2 Odom.t. 1NT 450 1000 5 1500 4000 3500 2924 Som.t.435T 3289150 2500 2000 b 12.56 %T Sample OS By CHEM User Date Tuesday, October 20 2021 cm-1 Exercises Draw the structures of the complexes prepared in Parts b) and c). Consult the IR spectra obtained. Given that >N-H bonds give rise to IR bands (NH) in the region 3000 to 3400 cm' and that >C=N-bonds give rise to IR bands (VC=N) in the region 1550 to 1600 cm-', what evidence do the spectra provide for the formation of a nickel complex from the ligand in parts a) and b)? und Comment on the the following experiments, nickel complexes of two isomeric ligands derived from NMR, MS and IR spectral measurements can be used to investigate the structures of diaminopropane and Pyrrole-2-aldehyde are prepared using different methods. 'H these compounds Experimental a) A Schiff base ligand from 1,3-diaminopropane and pyrrole-2-aldehyde This reaction is to be carried out in the designated fume cupboard. Dissolve pyrrole-2- aldehyde (0.95 g) in ethanol (5 cm) in a round-bottomed "Quickfit flask (100 cm). a graduated pipette, add 1,3-diaminopropane (0.40 cm) to the solution and mix Using the liquids, Fit a reflux condenser and warm the flask and reflux for 3-4 min and then stand in an ice bath for 2 hours. The mixture may solidify to a crystalline massor remain liquid. If a solid has deposited on cooling, collect this by filtration and wash it with a few cm of cold BOH. The combined filtrate and washing may deposit more products, as crystalline needles, on standing. If the mixture remains liquid, reduce the volume on a rotary evaporator, until solid starts to appear and then stand the flaskin an ice bath to complete the crystallization. Proceed to collect the product as described above. Allow the product to air dry and record your yicid. Obtain the IR (KBr disc). 'H NMR and mass spectra of your product. b) A nickel(II) complex form the Schiff base ligand Dissolve a portion (0.5 g) of the ligand prepared above is a) in warm ethanol (10 em Slowly add a solution of nickel ethanoate (nickel acetate, Ni(OCOCH3)2-4H-0,0.58) in water (10 cm) to produce a turbid, brick red mixture. Next add a solution of sodium carbonate (0.2 g) in water (5 cm) and stir the mixture for 20 minutes. After this time, collect the crude product by filtration and wash it with a little ethanol-water mixture (1:1, a few cm). Redissolve the red product in dichloromethane (ca. 40 cm) and dry the solution over a linic anhydrous magnesium sulfate. Remove the magnesium sulfate by filtration and wash it with a little dichloromethane. Add light- petroleum (80-100C, 40 cm) to the combined washings and filtrate, then remove the dichloromethane using a rotary evaporator (use a room temperature water bath, do not heat the flask). The red product will precipitate from the light petroleum as the dichloromethane is removed. The product may be collected by filtration and air dried. Record your yield and obtain the IR (KBr disc), 'H NMR and mass spectra of your product cm OR c) A nickel(II) complex with 1,2-diaminopropane and pyrrole-2-aldehyde equipped with a twin-necked adaptor, reflux condenser and dropping funnel. Place In the designated fume cupboard, set up a round-bottomed "Quickfit" flask (100 ethanol-water mixture (1:1 w/w. 50 cm) in the flask along with pyrrole-2-aldehyde anti-bumping granules. Heat the flask to dissolve the nickel ethanoate (a turbid rather (0.95 g), nickel ethanoate (nickel acetate, Ni(OCOCH3)2-4H20, 1.25 g) and 3 than clear solution will form) and then add an aqueous solution of NaOH (10% wiv, refluxing suspension of nickel hydroxide and aldehyde. Next, add water (10 cm) Add this diamine solution dropwise, over a period of about 20 minutes, love cm). Dissolve 1,2-diaminopropane (0.4 cm') in water (20 cm') in the dropping fuel with a little ethanol-water (1:1). Redissolve the product in dichloromethane (ca. 4) allow the mixture to cool. Collect the crude orange product by filtration and wash cm') while still in the filter funnel and allow the orange dichloromethane solution magnesium sulfate with a little dichloromethane. Add light-petroleum (80-100C) to magnesium sulfate, remove the magnesium sulfate by filtration and wash the filter into a clean conical flask (100 cm'). Dry this solution with a little anhydrous the combined filtrate and washings, and remove the dichloromethane using a rovary evaporator. (Use a room temperature water bath and do not heat the flask). The orange product will precipitate from the light-petroleum as the dichloromethane is removed and your yield may then be collected by filtration and allowed to dry in air. Record and obtain the IR (KBr disc), 'H NMR and mass spectra of your product. wo - Analyst Dale CHEMU Tuesday, October 26, 2021 19:03 PM Secondary 75 70 un 05 M. 1578AOSAT 60 55 50 1922 1941 45 40 301 7cm 1.4 T %T 35 N77 cm.1, 30.204T 30 25 20 N-H berts, 1450 35cm, 18.54%T qual > Najol. 15 TO 450 1000 1500 5 28833454 VAN -0- 2010 4000 3500 302891.60 2500 2000 cm-1 sample 7.73 %T Sample 003 BY CHEM User Date Tuesday, October 26 2021 CHEMU Tuesday, October 26, 2021 11:50 AM Anh Date 44 40 38 2. GAN 23 NO.254 1491 10,355 150,32225 1441 m2 tons SANT BY OST 36 34 32 3040m 1 an. 30 28 26 24 22 2 2043 1114 1145T 300 T2850 200LT 101 m: 1.27 14213-1.27 239 To stom-1.25 67% %T 254 25543T Buuhan A- bend CEN 77 band 1638mm 20 18 1000 450 16 14 12 16376cm-1, 11.51%T 11 4000 3500 362891.60 2500 2000 1500 cm-1 CHEM User 02_1 34 21 %T Sample 002 By CHEM User Date Tuesday, October 26 2021 How to CHEMU Tedy 2021150 PM Analys Date 54 50 YMT 45 1015 m., 1925T 40 35 11.30 4T 30 %T 1.1.2454 25 20 3436cm N-H band 15 Lords 1594am 10 2 Odom.t. 1NT 450 1000 5 1500 4000 3500 2924 Som.t.435T 3289150 2500 2000 b 12.56 %T Sample OS By CHEM User Date Tuesday, October 20 2021 cm-1 Exercises Draw the structures of the complexes prepared in Parts b) and c). Consult the IR spectra obtained. Given that >N-H bonds give rise to IR bands (NH) in the region 3000 to 3400 cm' and that >C=N-bonds give rise to IR bands (VC=N) in the region 1550 to 1600 cm-', what evidence do the spectra provide for the formation of a nickel complex from the ligand in parts a) and b)? und Comment on the