Question
In 2009, it was reported that a Mo-Mo quintuple bond (10, 2T, and 25 bonds) could be formed by reducing a quadruple-bonded dimolybdenum amidinate
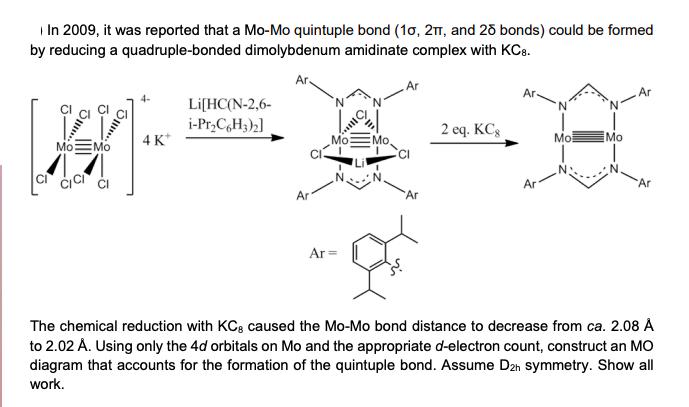
In 2009, it was reported that a Mo-Mo quintuple bond (10, 2T, and 25 bonds) could be formed by reducing a quadruple-bonded dimolybdenum amidinate complex with KC8. Ar. Ar Ar- 4- Li[HC(N-2, 6- i-PrC6H3)2] 2 eq. KCg Mo Mo 4K* Mo Mo. Mo: Mo Ar Ar Ar Ar= Ar Ar The chemical reduction with KCg caused the Mo-Mo bond distance to decrease from ca. 2.08 to 2.02 A. Using only the 4d orbitals on Mo and the appropriate d-electron count, construct an MO diagram that accounts for the formation of the quintuple bond. Assume D2h symmetry. Show all work.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Here is the stepbystep working to construct the MO diagram for the MoMo quintuple bond 1 We a...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Management Accounting Information for Decision-Making and Strategy Execution
Authors: Anthony A. Atkinson, Robert S. Kaplan, Ella Mae Matsumura, S. Mark Young
6th Edition
137024975, 978-0137024971
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



