Answered step by step
Verified Expert Solution
Question
1 Approved Answer
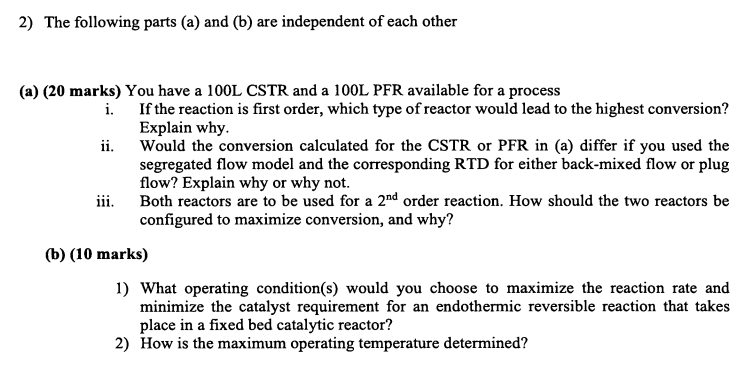
The following parts ( a ) and ( b ) are independent of each other ( a ) ( 2 0 marks ) You have
The following parts a and b are independent of each other
a marks You have a L CSTR and a L PFR available for a process
i If the reaction is first order, which type of reactor would lead to the highest conversion?
Explain why.
ii Would the conversion calculated for the CSTR or PFR in a differ if you used the
segregated flow model and the corresponding RTD for either backmixed flow or plug
flow? Explain why or why not.
iii. Both reactors are to be used for a order reaction. How should the two reactors be
configured to maximize conversion, and why?
b marks
What operating conditions would you choose to maximize the reaction rate and
minimize the catalyst requirement for an endothermic reversible reaction that takes
place in a fixed bed catalytic reactor?
How is the maximum operating temperature determined?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


