Question
The following PEC diagram describes the potential energy and number of configurations of the unmixed state (UM : Nal(s) + H20(1)) and mixed state
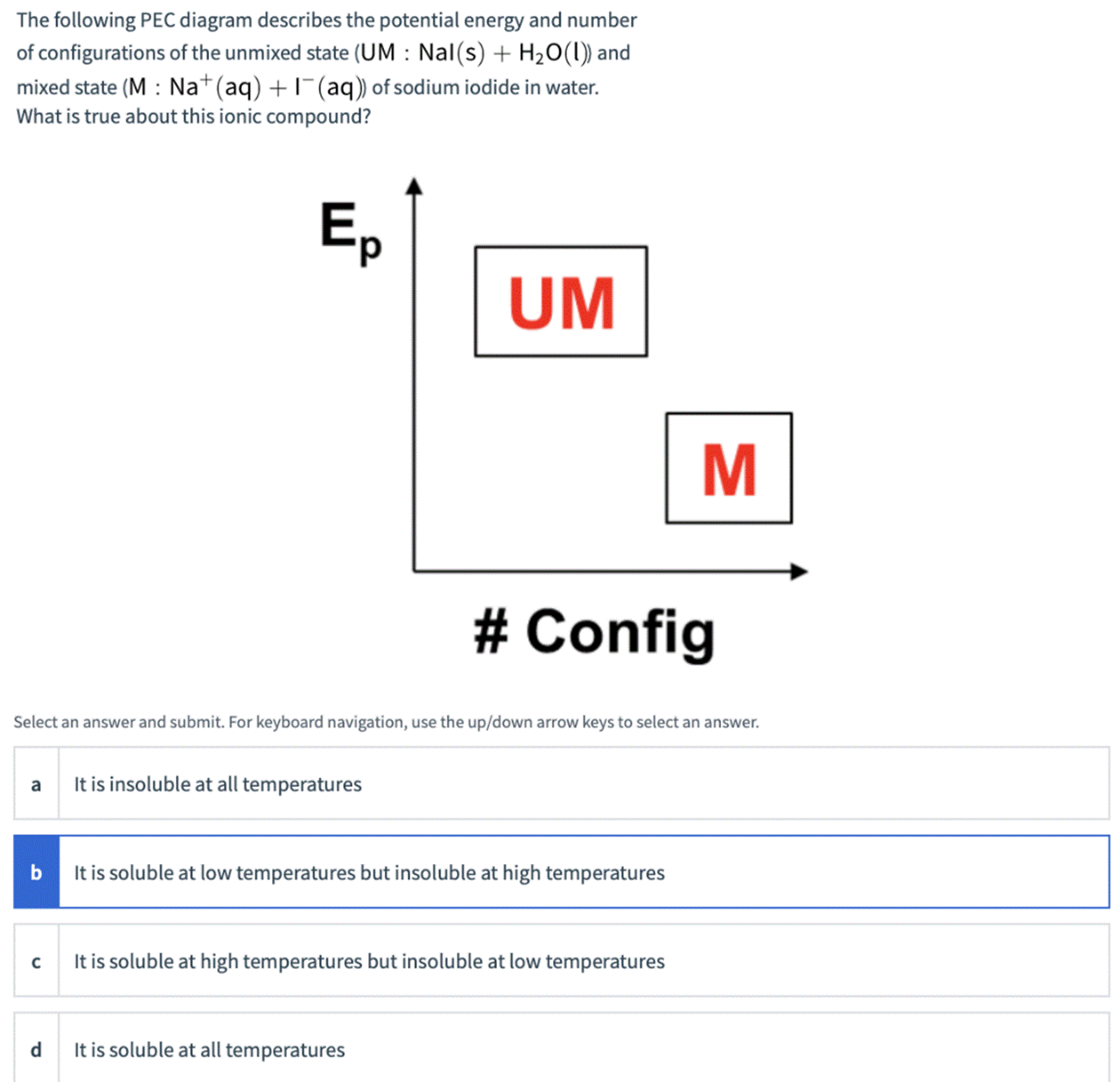
The following PEC diagram describes the potential energy and number of configurations of the unmixed state (UM : Nal(s) + H20(1)) and mixed state (M : Na+(aq) +1(aq)) of sodium iodide in water. What is true about this ionic compound? d. UM M # Config Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. It is insoluble at all temperatures It is soluble at low temperatures but insoluble at high temperatures It is soluble at high temperatures but insoluble at low temperatures d. It is soluble at all temperatures
Step by Step Solution
3.40 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
Option D is correct It is soluble at all temp...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Operations Management
Authors: William J Stevenson
12th edition
2900078024107, 78024102, 978-0078024108
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



