Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The following questions pertain to an electrolyte of 0.5 M Na2SO4 at 25 C. a. Find the molar conductivity of the solution and transference
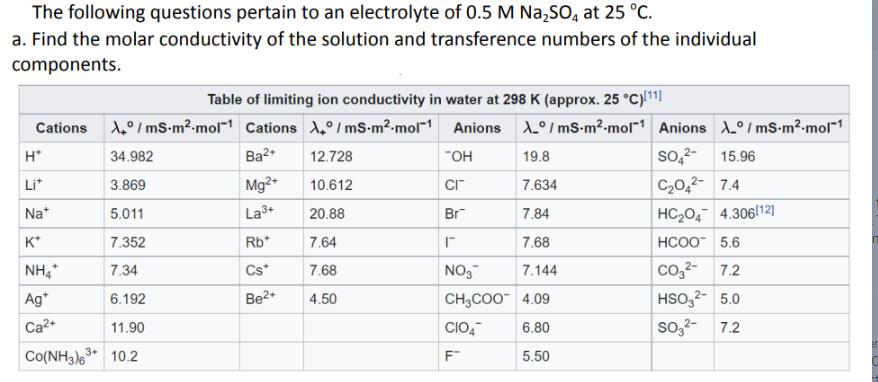

The following questions pertain to an electrolyte of 0.5 M Na2SO4 at 25 C. a. Find the molar conductivity of the solution and transference numbers of the individual components. Table of limiting ion conductivity in water at 298 K (approx. 25 C)[11] Cations /mS-m-mol Cations A/mS-m.mol-1 Anions A/mS-m-mol Anions A/mS-m.mol-1 SO42- 15.96 H* 34.982 Ba2+ 12.728 -OH 19.8 Li* 3.869 Mg2+ 10.612 CI 7.634 C2O42 7.4 Na+ 5.011 La 3+ 20.88 Br 7.84 HC2O4 4.306112] K* 7.352 Rb* 7.64 7.68 HCOO 5,6 NH4* 7.34 Cs* 7.68 NO3 7.144 CO2 7.2 Ag* 6.192 Be+ 4.50 CH3COO 4.09 HSO32 5.0 Ca2+ 11.90 CIO4 6.80 SO32 7.2 Co(NH3)63 10.2 3+ F 5.50 b. In a three-electrode setup, there is an uncompensated solution resistance between the reference electrode and the working electrode. What is the value of this solution resistance in 0.5 M NaSO if the distance between the reference and working electrodes is 3.5 cm and the cross-sectional area is 10 cm? c. If a total of 500 mV is applied by a potentiostat to your system and the total current is 20 mA, how much voltage is lost from the solution resistance? d. Qualitatively explain what would be the voltage drop if (i) the distance between the working and reference electrode was decreased? (ii) the concentration of the NaSO was increased?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


