Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The following reaction has been proposed to occur in two elementary steps. Overall Reaction: NO(g) + CO(g) NO(g) + CO(g) NO(g) + NO(g) NO3(g)

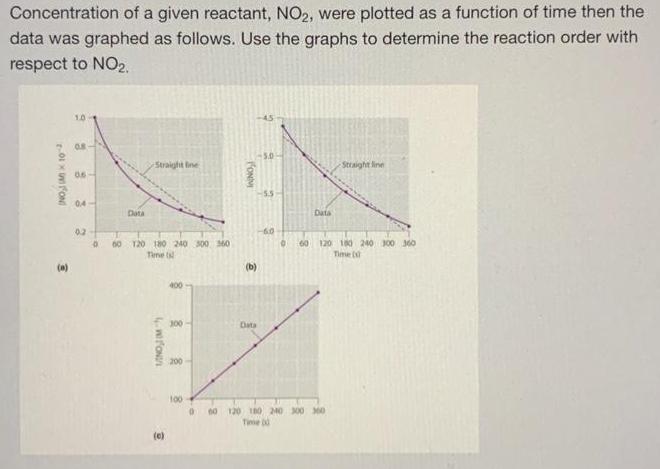
The following reaction has been proposed to occur in two elementary steps. Overall Reaction: NO(g) + CO(g) NO(g) + CO(g) NO(g) + NO(g) NO3(g) + NO(g) (Fast) NO3(g) + CO(g) NO(g) + CO(g) (Slow) - Step One Step Two Determine the rate law for this reaction. Show all of your work. Submit your answer on the last essay question as PDF or picture. Concentration of a given reactant, NO2, were plotted as a function of time then the data was graphed as follows. Use the graphs to determine the reaction order with respect to NO2. 9 a 2-01 FON 0.8 06- 04- 0.2 0 Data Straight line 60 120 180 240 300 360 Time t 4.WION/I (0) 400 300 200 100 FONMI (b) Data 50- 5.5 -60 0 60 Data Straight line 120 180 240 300 360 Time ( 000 120 180 240 300 360 Time d
Step by Step Solution
★★★★★
3.43 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
Given overall reaction Nog 9 CO 9 NO9 0 9 Shep 1 No 9 NO ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


