Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The following reaction was set-up as follows: R-OH HBr R-Br Where R-OH could be one of the following possible compounds 1-4 5.0 mL of
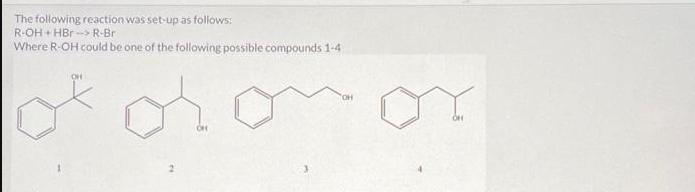
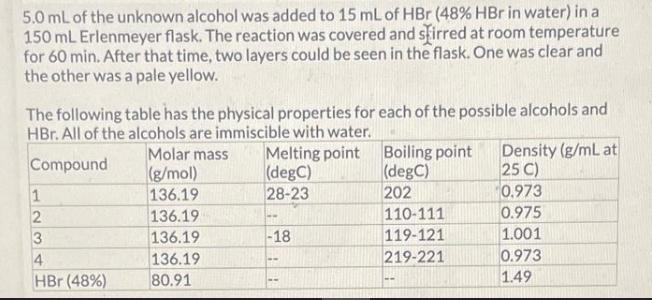

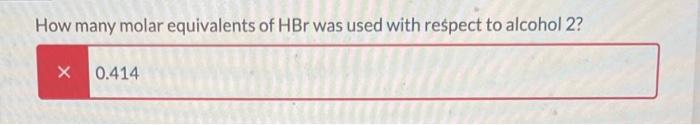
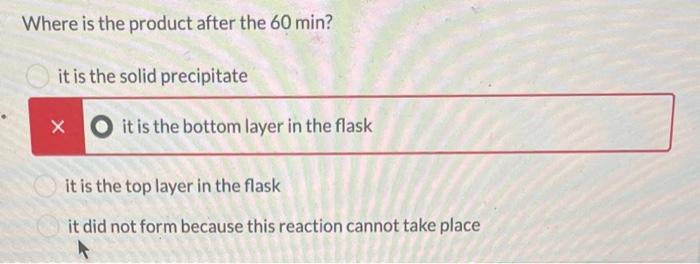

The following reaction was set-up as follows: R-OH HBr R-Br Where R-OH could be one of the following possible compounds 1-4 5.0 mL of the unknown alcohol was added to 15 mL of HBr ( 48% HBr in water) in a 150 mL Erlenmeyer flask. The reaction was covered and stirred at room temperature for 60 min. After that time, two layers could be seen in the flask. One was clear and the other was a pale yellow. The following table has the physical properties for each of the possible alcohols and HBr. All of the alcohols are immiscible with water. Compound 1 2 3 4 HBr (48%) Molar mass Melting point (g/mol) (degC) 136.19 28-23 136.19 136.19 136.19 80.91 -18 -- Boiling point (degC) 202 110-111 119-121 219-221 -- Density (g/mL at 25 C) 0.973 0.975 1.001 0.973 1.49 What needs to be done to balance the equation shown? Add H2O to the reactants side X Remove H2O from the reactants side Add H2O to the products side Remove H2O from the products side How many molar equivalents of HBr was used with respect to alcohol 2? X 0.414 Where is the product after the 60 min? it is the solid precipitate X it is the bottom layer in the flask it is the top layer in the flask it did not form because this reaction cannot take place 13 2 points How would the product and the starting alcohol differ in IR? Both have aromatic C-H bonds but only the product will have C=C aromatic bonds in the IR The product should have sp3 C-H bonds and the alcohol will not The product should not have an O-H bond, but will still have the sp3 and aromatic C-H bonds The product should not have an O-H bond or the sp3 C-H bonds
Step by Step Solution
★★★★★
3.41 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
In the reaction ROH HBr RBr the compound ROH represents an alcohol and HBr is hydrobromic acid The reaction proceeds by replacing the hydroxyl group OH in the alcohol with a bromine atom Br resulting ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


