Answered step by step
Verified Expert Solution
Question
1 Approved Answer
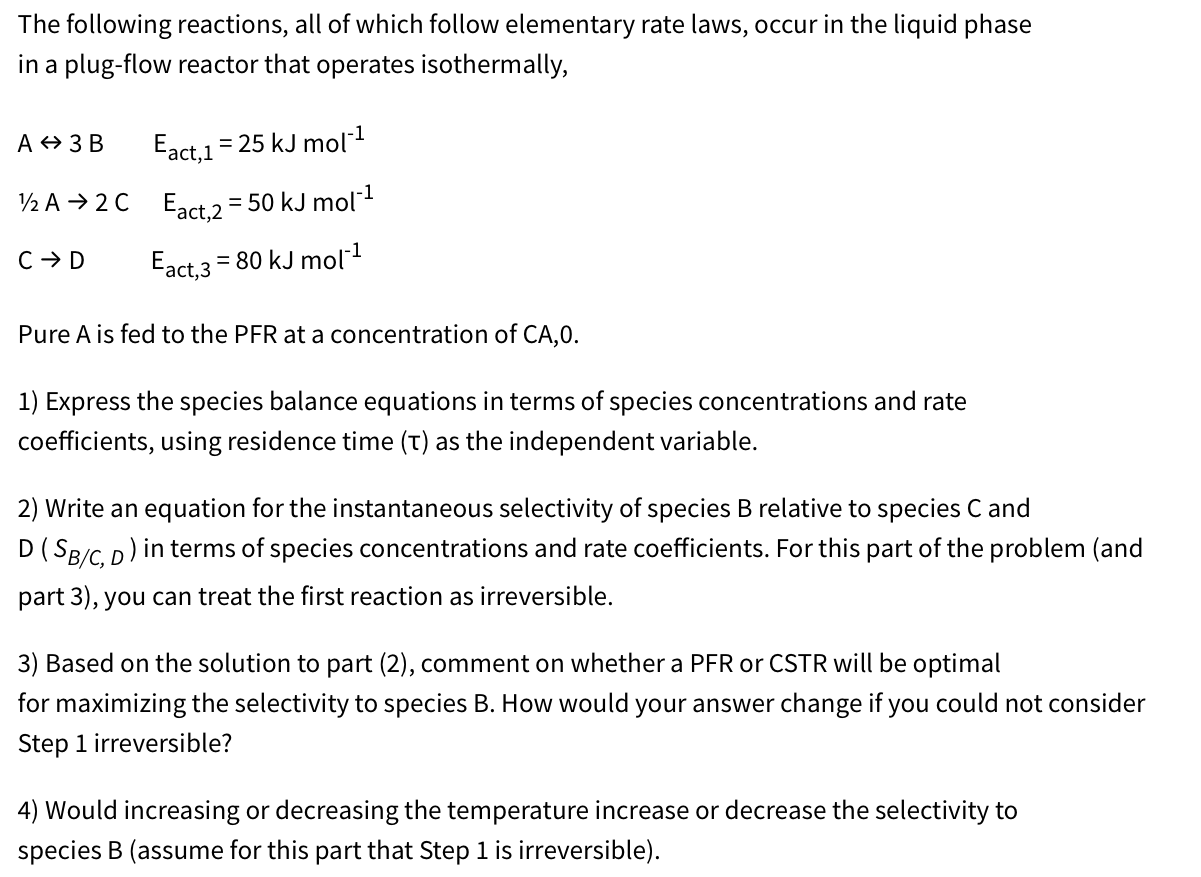
The following reactions, all of which follow elementary rate laws, occur in the liquid phase in a plug - flow reactor that operates isothermally, A
The following reactions, all of which follow elementary rate laws, occur in the liquid phase
in a plugflow reactor that operates isothermally,
Pure is fed to the PFR at a concentration of CA
Express the species balance equations in terms of species concentrations and rate
coefficients, using residence time as the independent variable.
Write an equation for the instantaneous selectivity of species relative to species and
in terms of species concentrations and rate coefficients. For this part of the problem and
part you can treat the first reaction as irreversible.
Based on the solution to part comment on whether a PFR or CSTR will be optimal
for maximizing the selectivity to species B How would your answer change if you could not consider
Step irreversible?
Would increasing or decreasing the temperature increase or decrease the selectivity to
species B assume for this part that Step is irreversible
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


