Question
The following table shows the time data run at different temperatures as described in Step 5 of the experimental procedure. Copy the concentration data from
The following table shows the time data run at different temperatures as described in Step 5 of the experimental procedure. Copy the concentration data from Reaction Mixture 1 above, and calculate the rate of each trial.
Reaction Mixture | [I-]o | [BrO3-]o | [H+]o | Temp. | Time | Rate |
1 | 40.0 oC | 44 sec | ||||
1 | 7.0 oC | 371 sec | ||||
1 | 1.0 oC | 480 sec |
Reaction Mixture | [I-]o | [BrO3-]o | [H+]o | Temp. | Time | Rate |
1 | 0.0020M | 0.008M | 0.02M | 19.0 oC | 182 sec | 1.0989x10-6Ms-1 |
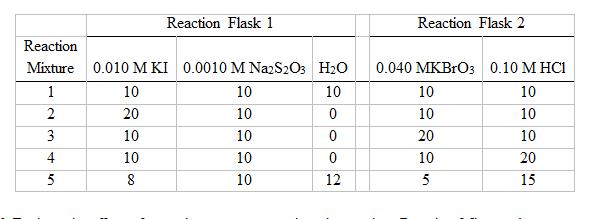
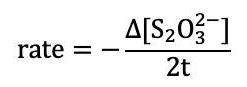
Reaction Flask 1 Reaction Flask 2 Reaction Mixture 0.010 M KI 0.0010 M NazS2Os H2O 0.040 MKBrO3 0.10 M HCI 10 10 10 10 10 20 10 10 10 3 10 10 20 10 4 10 10 10 20 5 8 10 12 5 15 12
Step by Step Solution
3.31 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Matching Supply with Demand An Introduction to Operations Management
Authors: Gerard Cachon, Christian Terwiesch
3rd edition
73525200, 978-0073525204
Students also viewed these Economics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



