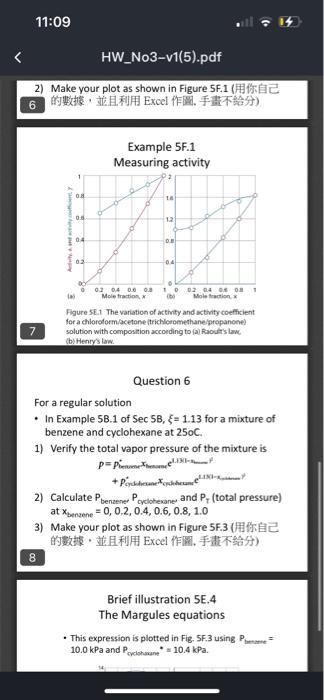
- The following temperature-composition data were obtained for a mixture of two liquids A and B at 1.00 atm, where x is the mole fraction in the liquid, and y the mole fraction in the vapor at equilibrium. The boiling points are 124C for A and 155C for B. Plot the temperature-composition for the mixture. What is the composition for the vapor in the equilibrium with the liquid of composition (1) xA=0.30,(2)x2 =0.15 Question 2 Based on the result of Question 1 , answer the following question: 1) If the initial solution is at x2=0.15@0=135C, how many theoretical plates needed to get the condensed solution with xa=0.9 ? 2) If the initial solution is at x4=0.35@0=125C, how many theoretical plates needed to get the condensed solution with xh=0.95? 3. Question 3 (1) What is activity? (2) What is activity coefficient? From Example 5F.1, Use the following information to calculate the activity and activity coefficient of trichioromethane (chloroform, C) in propanone (acetone, A) at 25C, treating it first as a solvent and then as a solute. 5 Question 5 From Raoult's law (chloroform regarded as the solvent) From Henry's law (chloroform regarded as the solute) 2) Make your plot as shown in Figure 5F.1 ( Example 5F.1 Measuring activitv 2) Make your plot as shown in Figure SF.1 ( Example 5F.1 AAsorurinn artinitu Figure 5 E.1. The variation of intinty and activity coefficient for a chloroform/acetone itrichloromethaneipropanone? solution with composition accoeding to (al faoult's lame. (b) Henry's law. Question 6 For a regular solution - In Example 5B.1 of Sec5B,=1.13 for a mixture of benzene and cyclohexane at 250C. 1) Verify the total vapor pressure of the mixture is +pcollinanexofhhrume1.100-x 2) Calculate PbenzenePcyclobexane and PT (total pressure) at xbenzene=0,0.2,0.4,0.6,0.8,1.0 3) Make your plot as shown in Figure 5F.3 ( 8 Brief illustration 5E.4 The Margules equations - This expression is plotted in Fig. SF.3 using P Plmene = 10.0kPa and Psedohmene=10.4kPa. Flgure SET The variation of activity and activity coefficient for a chloroformacetone itrichlocomethanefpropanone: 7 solution with composition accoeding to (al Raoults slawe. (b) Henry's lare. Question 6 For a regular solution - In Example 58.1 of Sec58,=1.13 for a mixture of benzene and cyclohexane at 250C. 1) Verify the total vapor pressure of the mixture is 2) Calculate PbensenePcycloherane and PT (total pressure) at xbensene=0,0.2,0.4,0.6,0.8,1.0 3) Make your plot as shown in Figure SF.3 8 Brief illustration 5E.4 The Margules equations - This expression is plotted in Fig. 5F.3 using Pbusu= 10.0 kPa and Pociohane=10.4kPa. Figere lis.] The computid wapour petsmum matief for a