Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The gas phase decomposition of sulfuryl chloride at 600K SO2Cl2(g)SO2(g)+Cl2(g) is first order in SO2Cl2 with a rate constant of 2.80103min1. If an experiment is
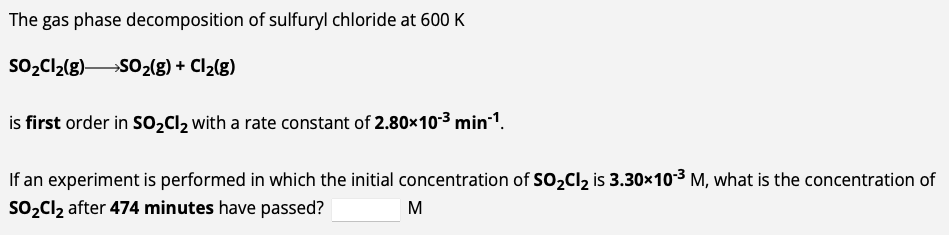
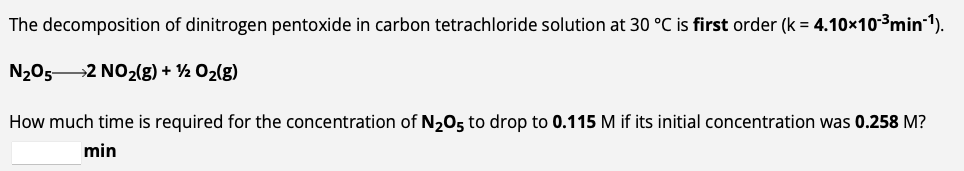
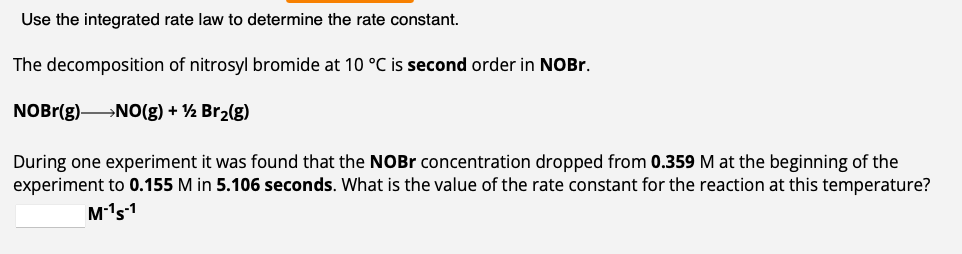
 The gas phase decomposition of sulfuryl chloride at 600K SO2Cl2(g)SO2(g)+Cl2(g) is first order in SO2Cl2 with a rate constant of 2.80103min1. If an experiment is performed in which the initial concentration of SO2Cl2 is 3.30103M, what is the concentration of SO2Cl2 after 474 minutes have passed? M The decomposition of dinitrogen pentoxide in carbon tetrachloride solution at 30C is first order (k=4.10103min1). N2O52NO2(g)+1/2O2(g) How much time is required for the concentration of N2O5 to drop to 0.115M if its initial concentration was 0.258M ? min Use the integrated rate law to determine the rate constant. The decomposition of nitrosyl bromide at 10C is second order in NOBr. NOBr(g)NO(g)+1/2Br2(g) During one experiment it was found that the NOBr concentration dropped from 0.359M at the beginning of the experiment to 0.155M in 5.106 seconds. What is the value of the rate constant for the reaction at this temperature? M1s1 Use the integrated rate law to calculate time for percent reacted. The gas phase decomposition of phosphine at 120C is first order (k=1.80102s1). PH3(g)1/4P4(g)+3/2H2(g) How much time is required for 78.7% of the PH3 intially present in a reaction flask to be converted to product at this temperature? S
The gas phase decomposition of sulfuryl chloride at 600K SO2Cl2(g)SO2(g)+Cl2(g) is first order in SO2Cl2 with a rate constant of 2.80103min1. If an experiment is performed in which the initial concentration of SO2Cl2 is 3.30103M, what is the concentration of SO2Cl2 after 474 minutes have passed? M The decomposition of dinitrogen pentoxide in carbon tetrachloride solution at 30C is first order (k=4.10103min1). N2O52NO2(g)+1/2O2(g) How much time is required for the concentration of N2O5 to drop to 0.115M if its initial concentration was 0.258M ? min Use the integrated rate law to determine the rate constant. The decomposition of nitrosyl bromide at 10C is second order in NOBr. NOBr(g)NO(g)+1/2Br2(g) During one experiment it was found that the NOBr concentration dropped from 0.359M at the beginning of the experiment to 0.155M in 5.106 seconds. What is the value of the rate constant for the reaction at this temperature? M1s1 Use the integrated rate law to calculate time for percent reacted. The gas phase decomposition of phosphine at 120C is first order (k=1.80102s1). PH3(g)1/4P4(g)+3/2H2(g) How much time is required for 78.7% of the PH3 intially present in a reaction flask to be converted to product at this temperature? S Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


