Answered step by step
Verified Expert Solution
Question
1 Approved Answer
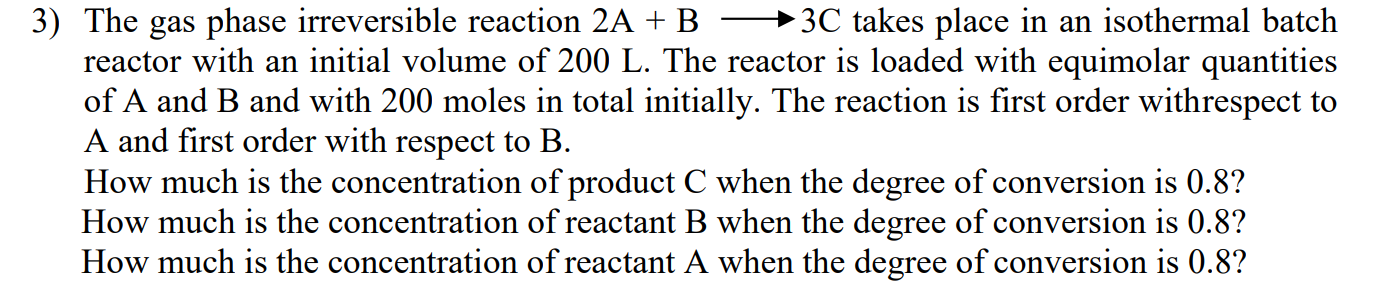
The gas phase irreversible reaction 2 A + Blongrightarrow 3 C takes place in an isothermal batch reactor with an initial volume of 2 0
The gas phase irreversible reaction Blongrightarrow takes place in an isothermal batch
reactor with an initial volume of The reactor is loaded with equimolar quantities
of A and B and with moles in total initially. The reaction is first order withrespect to
A and first order with respect to
How much is the concentration of product when the degree of conversion is
How much is the concentration of reactant when the degree of conversion is
How much is the concentration of reactant A when the degree of conversion is give me a clear answer
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


