Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The gas-phase reaction, A +0.5 B 3P occurs in a packed bed reactor. The feed composition consists of 80 mole % of A and
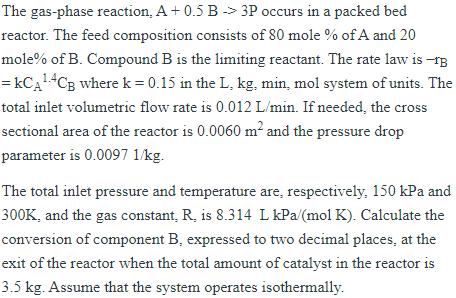
The gas-phase reaction, A +0.5 B 3P occurs in a packed bed reactor. The feed composition consists of 80 mole % of A and 20 mole% of B. Compound B is the limiting reactant. The rate law is -13 = KCA4CB where k = 0.15 in the L. kg. min., mol system of units. The total inlet volumetric flow rate is 0.012 L/min. If needed, the cross sectional area of the reactor is 0.0060 m and the pressure drop parameter is 0.0097 1/kg. The total inlet pressure and temperature are, respectively, 150 kPa and 300K, and the gas constant. R. is 8.314 L kPa/(mol K). Calculate the conversion of component B, expressed to two decimal places, at the exit of the reactor when the total amount of catalyst in the reactor is 3.5 kg. Assume that the system operates isothermally.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Step 1 Calculate the molar flow rate of component A at the inlet of the reactor To calculate the mol...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


