Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The given p - v diagram (not to scale) shows a closed system undergoing a power cycle using water. The cycle comprises of four
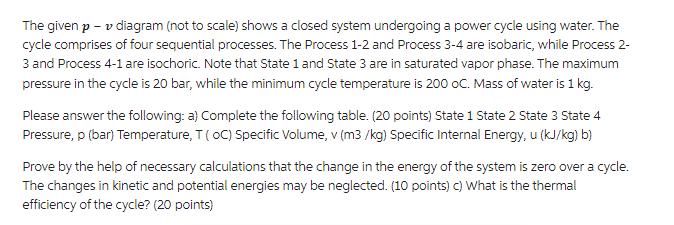
The given p - v diagram (not to scale) shows a closed system undergoing a power cycle using water. The cycle comprises of four sequential processes. The Process 1-2 and Process 3-4 are isobaric, while Process 2- 3 and Process 4-1 are isochoric. Note that State 1 and State 3 are in saturated vapor phase. The maximum pressure in the cycle is 20 bar, while the minimum cycle temperature is 200 oC. Mass of water is 1 kg. Please answer the following: a) Complete the following table. (20 points) State 1 State 2 State 3 State 4 Pressure, p (bar) Temperature, T (oC) Specific Volume, v (m3/kg) Specific Internal Energy, u (kJ/kg) b) Prove by the help of necessary calculations that the change in the energy of the system is zero over a cycle. The changes in kinetic and potential energies may be neglected. (10 points) c) What is the thermal efficiency of the cycle? (20 points)
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a Complete the following table State Pressure p bar Temperature T C Specific Volume v mkg Specific Internal Energy u kJkg 1 20 623 000106 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


