Question
The graph below shows the concentration of cyclobutane as it decomposes over time at 1270K. (i) Based on the graph, determine the order of the
The graph below shows the concentration of cyclobutane as it decomposes over time at 1270K.
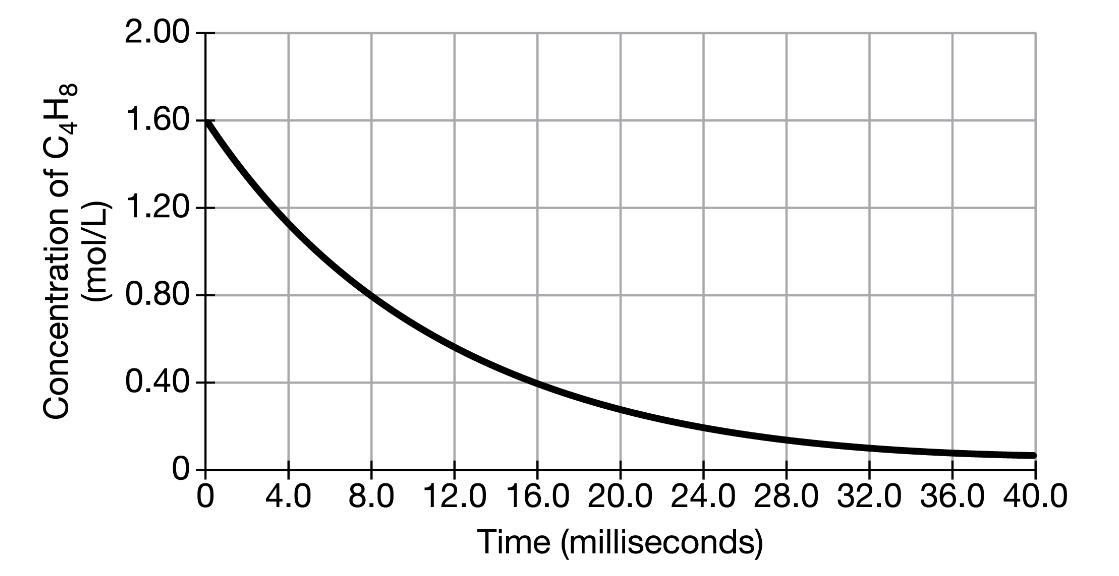
(i) Based on the graph, determine the order of the decomposition reaction of cyclobutane at 1270K. Justify your answer.
(ii) Calculate the time, in milliseconds, that it would take 99 percent of the original cyclobutane at 1270K to decompose.
2.00 1.60 1.20 0.80 0.40- 4.0 8.0 12.0 16.0 20.0 24.0 28.0 32.0 36.0 40.0 Time (milliseconds) Concentration of C,H3 (7/|ou)
Step by Step Solution
3.49 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Financial Algebra advanced algebra with financial applications
Authors: Robert K. Gerver
1st edition
978-1285444857, 128544485X, 978-0357229101, 035722910X, 978-0538449670
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



