Answered step by step
Verified Expert Solution
Question
1 Approved Answer
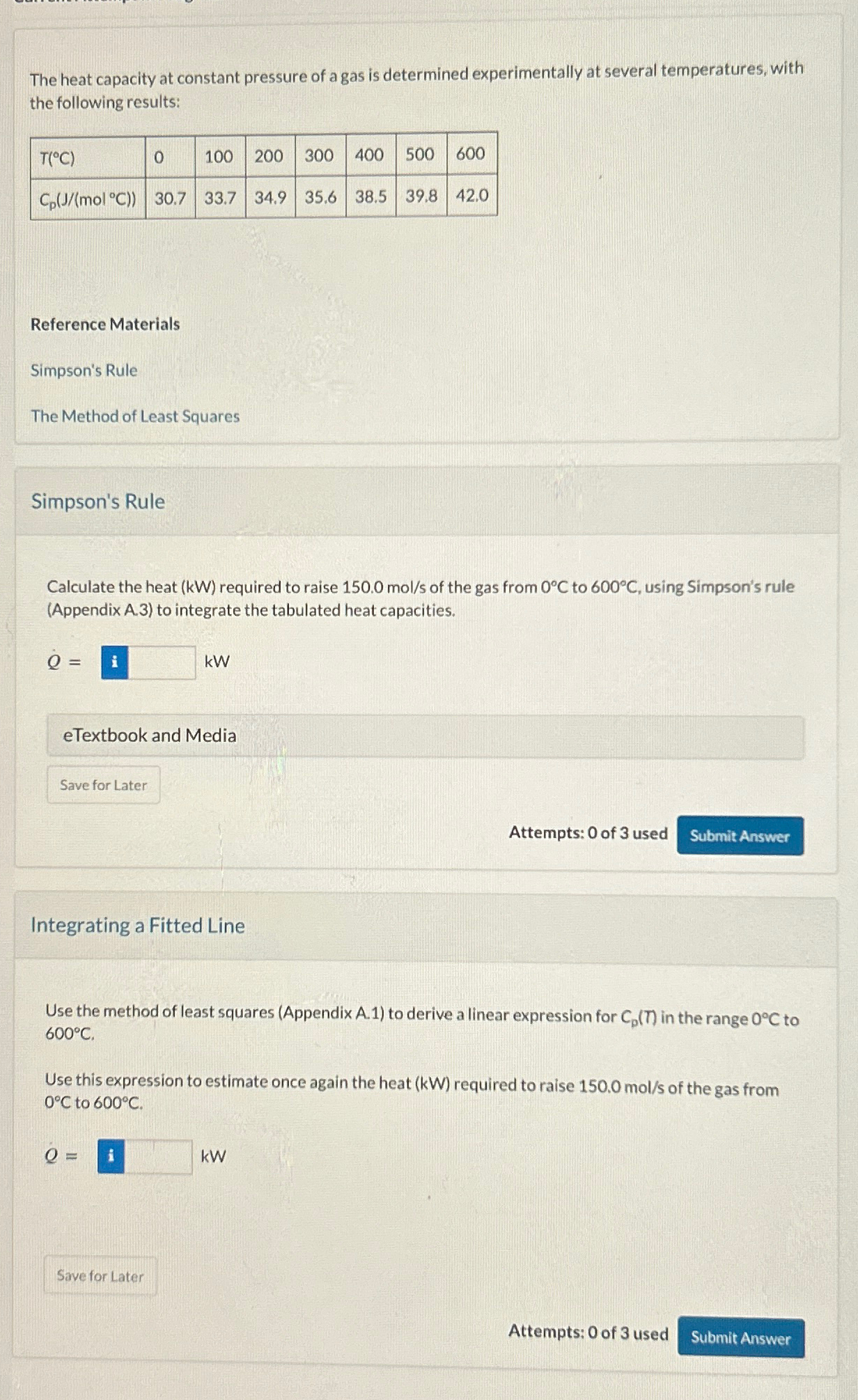
The heat capacity at constant pressure of a gas is determined experimentally at several temperatures, with the following results: table [ [ T (
The heat capacity at constant pressure of a gas is determined experimentally at several temperatures, with the following results:
table
Reference Materials
Simpson's Rule
The Method of Least Squares
Simpson's Rule
Calculate the heat required to raise of the gas from to using Simpson's rule Appendix A to integrate the tabulated heat capacities.
eTextbook and Media
Attempts: of used
Integrating a Fitted Line
Use the method of least squares Appendix A to derive a linear expression for in the range to
Use this expression to estimate once again the heat required to raise of the gas from to
Attempts: of used
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


